��Ŀ����
����Ŀ��ij��ѧѧϰС����̽����������ʣ��ü����������ʵ�飺
ʵ��1��![]()
ʵ��2����������ɫ��Һװ���ܷ��Ժõ���ɫ�Լ�ƿ�С���һ��ʱ�䣬��Һ��ɫ��dz����ƿ��ƿ�ڳ��ְ�����
ʵ��3��������ͼ��ʵ��װ�ý��м�����ѻ���
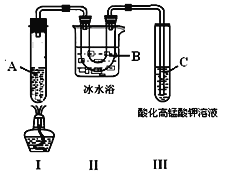
��ش��������⣺
��1����ʵ��1 ֪���������������______________��
��2��ʵ��2 �еij�ɫ��Һ��dz��ԭ����_______��
A���������巢������ȡ����Ӧ B���������Ϊ��ɫ����
C��Һ������ӷ�Ũ�Ƚ��� D��������Һ�巢���ӳɷ�Ӧ
E��Һ���뼺��ֲ㣬�ܶȴ��Һ�����²�
��3���Թ�C�п�����������____________________________��˵����_________���ɣ�
��4����д��װ�����з����б������ɵ��ѻ���Ӧ����ʽ��___________________________��
���𰸡������ˮ����������ˮ�����ܽ���AB���Ը�����ص���ɫ��ȥ��̬ϩ��CH3(CH2)4CH3![]() CH3CH2CH3��CH3CH=CH2
CH3CH2CH3��CH3CH=CH2
��������
(1)��ˮ�������Ϻ��²㼸������ɫ�ģ��ҷ�Һ��õ���ɫ��Һ��˵�������ˮ�ᡢ������ˮ�����ܽ��壻(2)���ڴ�ƿ��ƿ�ڳ��ְ�������˵�����廯�����ɣ��������������������������巢��ȡ����Ӧ������ɫ�廯����廯�⣬���ѡAB��(3)�����ѻ�����̬��ϩ�����ɣ�ϩ������̼̼˫������ʹ���Ը��������Һ��ɫ�����Թ�C�п��������������Ը�����ص���ɫ��ȥ��(4)��һ�������£������ѻ����ɱ���ͱ�ϩ����Ӧ�ķ���ʽΪ CH3(CH2)4CH3![]() CH3CH2CH3��CH3CH=CH2��
CH3CH2CH3��CH3CH=CH2��

����Ŀ��Ϊ�ᴿ��������(�����ڵ�����Ϊ����)����ѡ�õij����Լ��ͷ��뷽������ȷ����( )
ѡ�� | A | B | C | D |
���ᴿ������ | ˮ(ú��) | ������(ˮ) | �ױ�(ˮ) | �Ҵ�(����) |
�����Լ� | �� | NaOH��Һ | �� | NaOH��Һ |
���뷽�� | ��Һ | ���� | ���� | ��Һ |
A.AB.BC.CD.D