ΧβΡΩΡΎ»ί
Έε÷÷ΕΧ÷ήΤΎ‘ΣΥΊAΓΔBΓΔCΓΔDΓΔEΒΡ‘≠Ή”–ρ ΐ“ά¥Έ‘ω¥σΘ§AΚΆCΆ§ΉεΘ§BΚΆD Ά§ΉεΘ§CάκΉ”ΚΆBάκΉ”ΨΏ”–œύΆ§ΒΡΒγΉ”≤ψΫαΙΙΓΘAΚΆBΓΔDΓΔEΨυΡή–Έ≥…Ι≤Φέ–ΆΜ·ΚœΈοΓΘAΚΆB–Έ≥…ΒΡΤχΧε‘ΎΥ°÷–≥ Φν–‘Θ§CΚΆE–Έ≥…ΒΡΜ·ΚœΈο‘ΎΥ°÷–≥ ÷––‘ΓΘΜΊ¥πœ¬Ν–Έ ΧβΘΚ
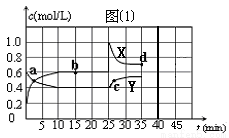
Θ®1Θ©Έε÷÷‘ΣΥΊ÷–Θ§‘≠Ή”ΑκΨΕΉν¥σΒΡ « Θ§Ζ«Ϋπ τ–‘Ήν«ΩΒΡ « Θ®Χν‘ΣΥΊΖϊΚ≈Θ©ΘΜ
Θ®2Θ©”…AΚΆBΓΔDΓΔEΥυ–Έ≥…ΒΡΙ≤Φέ–ΆΜ·ΚœΈο÷–Θ§»»Έ»Ε®–‘Ήν≤νΒΡ « Θ®”ΟΜ·―ß Ϋ±μ ΨΘ©ΘΜ
Θ®3Θ©AΚΆE–Έ≥…ΒΡΜ·ΚœΈο”κAΚΆB–Έ≥…ΒΡΜ·ΚœΈοΖ¥”ΠΘ§≤ζΈοΒΡΜ·―ß ΫΈΣ Θ§Τδ÷–¥φ‘ΎΒΡΜ·―ßΦϋάύ–ΆΈΣ ΘΜ
Θ®4Θ©DΉνΗΏΦέ―θΜ·ΈοΒΡΥ°Μ·ΈοΒΡΜ·―ß ΫΈΣ ΘΜ
Θ®5Θ©ΒΞ÷ EΒΡ‘≠Ή”ΫαΙΙ Ψ“βΆΦΈΣ ΘΜD‘Ύ≤Μ≥δΉψΒΡE÷–»Φ…’Θ§…ζ≥…ΒΡ÷ς“Σ≤ζΈοΒΡΜ·―ß ΫΈΣ ΘΜ
Θ®6Θ©ΒΞ÷ E”κΥ°Ζ¥”ΠΒΡάκΉ”ΖΫ≥Χ ΫΈΣ ΓΘ
Θ®1Θ©Na ΓΔ Cl
Θ®2Θ©PH3
Θ®3Θ©NH4Cl
Θ®4Θ©H3PO4
Θ®5Θ©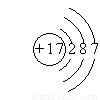 ΓΔPCl3
ΓΔPCl3
Θ®6Θ©Cl2+H2O=HClO+H++Cl-
ΓΨΫβΈωΓΩ
‘ΧβΖ÷ΈωΘΚAΚΆB–Έ≥…ΒΡΤχΧε‘ΎΥ°÷–≥ Φν–‘Θ§ΥΒΟςA «HΘ§B «N‘ΣΥΊΘΜAΓΔBΓΔCΓΔDΓΔEΒΡ‘≠Ή”–ρ ΐ“ά¥Έ‘ω¥σΘ§Υυ“‘C «NaΘ§D «P‘ΣΥΊΘΜCΚΆE–Έ≥…ΒΡΜ·ΚœΈο‘ΎΥ°÷–≥ ÷––‘Θ§E «Cl‘ΣΥΊΓΘ
Θ®1Θ©Έε÷÷‘ΣΥΊ÷–Θ§‘≠Ή”ΑκΨΕΉν¥σΒΡ «NaΘ§Ζ«Ϋπ τ–‘Ήν«ΩΒΡ «ClΘΜ
Θ®2Θ©BΓΔDΓΔE÷–Ζ«Ϋπ τ–‘Ήν»θΒΡ «PΘ§Υυ“‘”κA–Έ≥…ΒΡΤχΧ§«βΜ·Έο÷–Έ»Ε®–‘Ήν≤νΒΡPH3
Θ®3Θ©AΚΆE–Έ≥…ΒΡΜ·ΚœΈο «¬»Μ·«βΘ§AΚΆB–Έ≥…ΒΡΜ·ΚœΈο «Α±ΤχΘ§Εΰ’ΏΖ¥”Π…ζ≥…¬»Μ·οßΘ§Μ·―ß ΫΈΣNH4Cl
(4) DΉνΗΏΦέ―θΜ·ΈοΒΡΥ°Μ·Έο «ΝΉΥαΘ§Μ·―ß ΫΈΣH3PO4
Θ®5Θ©¬»ΒΡ‘≠Ή”ΫαΙΙ Ψ“βΆΦΈΣ Θ§P‘Ύ≤ΜΉψΒΡ¬»Τχ÷–»Φ…’Θ§…ζ≥…»ΐ¬»Μ·ΝΉΘ§Μ·―ß ΫΈΣPCl3
Θ§P‘Ύ≤ΜΉψΒΡ¬»Τχ÷–»Φ…’Θ§…ζ≥…»ΐ¬»Μ·ΝΉΘ§Μ·―ß ΫΈΣPCl3
Θ®6Θ©¬»ΤχΚΆΥ°Ζ¥”ΠΒΡάκΉ”ΖΫ≥Χ ΫΈΣCl2+H2O=HClO+H++Cl-
ΩΦΒψΘΚΩΦ≤ι‘ΣΥΊΆΤΕœΘ§‘≠Ή”ΑκΨΕΓΔΖ«Ϋπ τ–‘ΓΔΤχΧ§«βΜ·ΈοΒΡΈ»Ε®–‘ΓΔΜ·―ß ΫΓΔ‘≠Ή”ΫαΙΙ Ψ“βΆΦΓΔάκΉ”ΖΫ≥Χ ΫΒΡ≈–Εœ