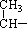ΧβΡΩΡΎ»ί
Θ®1Θ©Ρ≥ΚψΈ¬Κψ»ίΧθΦΰœ¬ΒΡΩ…ΡφΖ¥”ΠΘΚN2(g)+3H2(g)  2NH3(g) ΓςHΘΦ0Θ§Τπ Φ ≥δ»κΘαmol N2ΓΔΘβmol H2Θ§ ¥οΒΫΤΫΚβΚσΘ§N2ΓΔH2ΓΔNH3ΒΡΈο÷ ΒΡΝΩΖ÷±πΈΣ1 molΓΔ3 molΓΔ10 molΘ§ΜΊ¥πœ¬Ν–Έ ΧβΘΚ
2NH3(g) ΓςHΘΦ0Θ§Τπ Φ ≥δ»κΘαmol N2ΓΔΘβmol H2Θ§ ¥οΒΫΤΫΚβΚσΘ§N2ΓΔH2ΓΔNH3ΒΡΈο÷ ΒΡΝΩΖ÷±πΈΣ1 molΓΔ3 molΓΔ10 molΘ§ΜΊ¥πœ¬Ν–Έ ΧβΘΚ
ΔΌ A= Θ§ B= ΘΜ
ΔΎ ΤΫΚβΚσΘ§‘Ό≥δ»κ5 molNH3Θ§Μ·―ßΤΫΚβΫΪœρ “ΤΕ·Θ®ΧνΓΑΉσΓ±ΜρΓΑ”“Γ±ΜρΓΑ≤Μ“ΤΕ·Γ±Θ©Θ§¥ο–¬ΤΫΚβ ±N2ΒΡΑΌΖ÷Κ§ΝΩ ‘≠ΤΫΚβ ±N2ΒΡΑΌΖ÷Κ§ΝΩΘ®ΧνΓΑ¥σ”ΎΓ±ΜρΓΑ–Γ”ΎΓ±ΜρΓΑΒ»”ΎΓ±Θ©ΘΜ
ΔέΤΫΚβΚσ»τ…ΐΗΏΈ¬Ε»Θ§‘ρΤΫΚβœρ ΖΫœρ“ΤΕ·ΘΜ»τ‘ωΦ”―Ι«ΩΘ§‘ρΤΫΚβœρ
ΖΫœρ“ΤΕ·Θ®ΧνΓΑœρΉσΓ±ΜρΓΑœρ”“Γ±Θ©ΓΘ
Θ®2Θ©Ρ≥ΚψΈ¬Κψ―ΙΧθΦΰœ¬ΒΡΩ…ΡφΖ¥”ΠΘΚN2(g)+3H2(g)  2NH3(g)Θ§Τπ Φ≥δ»κ1 mol N2ΓΔ3 mol H2ΓΔ16 mol NH3Θ§»ίΤςΒΡ»ίΜΐΈΣV LΓΘ¥οΒΫΤΫΚβΚσΘ§N2ΓΔH2ΓΔNH3ΒΡΈο÷ ΒΡΝΩΖ÷±πΈΣAmolΓΔBmolΓΔc molΘ§¥Υ ±»ίΤςΒΡ»ίΜΐΈΣ1.1V LΘ§ΜΊ¥πœ¬Ν–Έ ΧβΘΚ
2NH3(g)Θ§Τπ Φ≥δ»κ1 mol N2ΓΔ3 mol H2ΓΔ16 mol NH3Θ§»ίΤςΒΡ»ίΜΐΈΣV LΓΘ¥οΒΫΤΫΚβΚσΘ§N2ΓΔH2ΓΔNH3ΒΡΈο÷ ΒΡΝΩΖ÷±πΈΣAmolΓΔBmolΓΔc molΘ§¥Υ ±»ίΤςΒΡ»ίΜΐΈΣ1.1V LΘ§ΜΊ¥πœ¬Ν–Έ ΧβΘΚ
ΔΌ Τπ Φ ±Ζ¥”ΠΥΌ¬ V’ΐ VΡφΘ®ΧνΓΑ¥σ”ΎΓ±ΜρΓΑ–Γ”ΎΓ±ΜρΓΑΒ»”ΎΓ±Θ©
ΔΎ ΤΫΚβ ±A= Θ§B= ΓΘ
Δέ »τΤΫΚβΚσ‘Ό≥δ»κ3.6 molNH3Θ§÷Ί–¬Ϋ®ΝΔΤΫΚβ ±»ίΤςΒΡ»ίΜΐΈΣ LΓΘ
(10Ζ÷)(1) ΔΌ 6, 18 ΔΎ Ήσ.–Γ”Ύ Δέ œρΉσΓΔœρ”“
(2) ΔΌ –Γ”Ύ.ΔΎ 2 6 Δέ 1.32V
ΓΨΫβΈωΓΩ
‘ΧβΖ÷ΈωΘΚΘ®1Θ©¥οΒΫΤΫΚβΚσΘ§N2ΓΔH2ΓΔNH3ΒΡΈο÷ ΒΡΝΩΖ÷±πΈΣ1 molΓΔ3 molΓΔ10 molΘ§Ζ¥”ΠœϊΚΡN2ΓΔH2ΒΡΈο÷ ΒΡΝΩΖ÷±πΈΣ5molΓΔ15molΘ§Υυ“‘ΔΌa=6ΓΔb=18ΘΜΔΎΚψΈ¬Κψ»ίΧθΦΰœ¬Θ§‘Ό≥δ»κ5 molNH3Θ§Α±Τχ≈®Ε»‘ω¥σΘ§ΤΫΚβœρΉσ“ΤΕ·ΘΜ»τΫΪ»ίΤςΧεΜΐΥθ–Γ“ΜΑκΘ§≥δ»κ5 molNH3Θ§”κ‘≠ΤΫΚβΒ»–ßΘ§ΫΪΝΫΗωΤΫΚβΚœΕΰΈΣ“ΜΘ§Μ÷Η¥ΈΣ‘≠»ίΤςΒΡΧεΜΐΘ§‘ρΤΫΚβΜα’ΐœρ“ΤΕ·Θ§ΒΣΤχΒΡΑΌΖ÷Κ§ΝΩΦθ–ΓΘ§ΒΪΉΣΜ·¬ ‘ω¥σΘΜΔέ…ΐΈ¬Θ§ΤΫΚβœρΈϋ»»Ζ¥”ΠΖΫœρ“ΤΕ·Θ§Υυ“‘…ΐΈ¬Θ§Μ·―ßΤΫΚβœρΉσ“ΤΕ·Θ§‘ω¥σ―Ι«ΩΘ§ΤΫΚβœρ―Ι«ΩΦθ–ΓΒΡΖΫœρ“ΤΕ·Θ§“ρ¥Υœρ”““ΤΕ·ΓΘ
Θ®2Θ©ΔΌΚψΈ¬Κψ―ΙΧθΦΰœ¬Θ§ΤΫΚβΚσ»ίΤςΧεΜΐ‘ω¥σΘ§ΥΒΟςΤχΧεΈο÷ ΒΡΝΩ‘ωΦ”Θ§Ζ¥”ΠΡφœρΫχ––V’ΐ< VΡφΔΎΖ¥”ΠΡφœρΫχ––Θ§ΤΫΚβΚσΤχΧεΉήΈο÷ ΒΡΝΩΈΣ20ΓΝ1.1=22mol,…η…ζ≥…ΒΣΤχxΘ§‘ρ«βΤχΈΣ3x,œϊΚΡΑ±Τχ2x,Ω…ΒΟΘΚ1+x+3+3x+16-2x=22,ΫβΒΟx=1,Υυ“‘a=1+x-2,b=3+3x=6,c=16-2x=14
ΔέΤπ Φ≥δ»κ1 mol N2ΓΔ3 mol H2ΓΔ16 mol NH3Θ§œύΒ±”Ύ≥δ»κ18mol NH3Θ§ΤΫΚβ ±ΧεΜΐΈΣ1.1V,‘ΎΚψΈ¬Κψ―ΙΧθΦΰœ¬Θ§≥δ»κ3.6mol NH3Θ§ΤΫΚβ ±ΒΡΧεΜΐΈΣ3.6/18ΓΝ1.1V=0.22V,Υυ“‘ΉήΧεΜΐΈΣΘΚ1.1V+0.22V=1.32V
ΩΦΒψΘΚΩΦ≤ιΜ·―ßΤΫΚβΒΡ“ΤΕ·ΓΔ≤ΜΆ§ΧθΦΰΒΡΒ»–ßΤΫΚβΦΑœύΙΊΦΤΥψ