��Ŀ����
����Ŀ��ijͬѧ��Cu�ij�������������ʽ���ʵ��̽�����о�������������£�
(1)Ϊ̽��Cu(OH)2�Ƿ��Al(OH)3һ���������ԣ���ѡ��Cu(OH)2�⣬����ѡ�õ��Լ�Ϊ____________ (�����) a����ˮ b������������Һ c��ϡ���� d��������
(2)Ϊ̽����ͬ��̬ͭ���ȶ��ԣ���������ʵ�飺
�ٽ�CuO��ĩ������1000��������ȫ�ֽ�ɺ�ɫ��Cu2O��ĩ��
����Cu2O�м�����ϡ���ᣬ�õ���ɫ��Һ��һ�ֺ�ɫ���壬�÷�Ӧ�����ӻ�ѧ����ʽΪ ____________ ��
(3)Ϊ��̽�������ܷ������һ����ԭCuO�����������ṩ������װ�ý���ʵ��(�г�װ��δ��)��װ��A�����������������������Ӹ������ӿڣ�˳��Ϊa��___��___��___��___��___��___��h
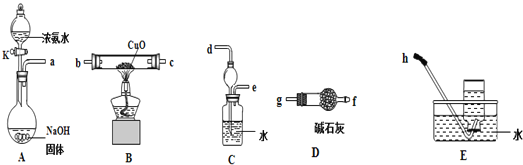
��ʵ�鿪ʼʱ����Һ©���Ļ���K������Ũ��ˮû�е��£�������������ܾ�û�ж���������ܵ�ԭ����_______________________________________��
��ʵ�������װ��B�й����ɺ�ɫ��Ϊ��ɫ��װ��E���ռ�����ɫ��ζ�����壬�ڿ���������ɫ�仯����ȼ��þ������������ȼ�գ���Bװ���з����Ļ�ѧ����ʽΪ_____________________��
��װ��C�е�����װ�õ�������_________________��
���𰸡�bc Cu2O+2H+��Cu2++Cu+H2O g f b c d e û�н���Һ©�����ϵIJ�������(��ʹ���ϵİ��ۣ���С�ף���©���ϵ�С��) 3CuO+2NH3![]() 3Cu +N2+3H2O ������
3Cu +N2+3H2O ������
��������
(1)֤��Al(OH)3�����Ե��Լ���ǿ���ǿ����Ҫ��֤��Cu(OH)2�����ԣ�Ҳ����ѡǿ���ǿ����Һ��������ǿ�ᣬ���Կ���ѡȡ������������ǿ����Կ�ѡȡ����ˮ��������������ᣬ������ѡ������bc��ȷ���ʴ�Ϊ��bc��
(2)����Cu2O�м�����ϡ���ᣬ�õ���ɫ��Һ��һ�ֺ�ɫ���壬��˵��������ͭ��ϡ���ᷴӦ���ɵ�������ͭ��ˮ�͵���ͭ����˸÷�Ӧ�����ӻ�ѧ����ʽΪ��Cu2O+2H+=Cu2++Cu+H2O���ʴ�Ϊ��Cu2O+2H+=Cu2++Cu+H2O��
(3)Aװ�����Ʊ������ģ����ɵİ����к���ˮ��������Ҫͨ����ʯ�Ҹ������Ϊ������������ˮ�������İ����ڽ���β������ʱ��Ҫ��ֹ������������ȷ������˳��Ϊ��a��g��f��b��c��d��e��h���ʴ�Ϊ��g��f��b��c��d��e��
�����ڷ�Ӧ���ɰ������ɣ���ƿ��ѹǿ���°�ˮ���ܵ��£��������ܵ�ԭ����û�н���Һ©�����ϵIJ�������(��ʹ���ϵİ���(��С��)��©���ϵ�С��)���ʴ�Ϊ��û�н���Һ©�����ϵIJ�������(��ʹ���ϵİ���(��С��)��©���ϵ�С��)��
��ʵ�������װ��B�й����ɺ�ɫ��Ϊ��ɫ����˵���е���ͭ���ɣ�װ��E���ռ�����ɫ��ζ�����壬�ڿ���������ɫ�仯����ȼ��þ������������ȼ�գ���˸�����Ӧ���ǵ���������Bװ���з����Ļ�ѧ����ʽΪ3CuO+2NH3![]() 3Cu+N2+3H2O���ʴ�Ϊ��3CuO+2NH3
3Cu+N2+3H2O���ʴ�Ϊ��3CuO+2NH3![]() 3Cu+N2+3H2O��
3Cu+N2+3H2O��
�����ڰ�����������ˮ��ֱ��ͨ��ˮ�����գ�������������װ��C�е�����װ�õ������Ƿ��������ʴ�Ϊ����������