ΧβΡΩΡΎ»ί
“ΜΑψ≤βΕ®―υΤΖ÷–≥…Ζ÷Κ§ΝΩΒΡ Β―ι”Π÷ΊΗ¥2ΓΪ3¥ΈΓΘΈΣΝΥ≤βΕ®Ρ≥«β―θΜ·ΡΤΙΧΧε÷–Μλ”–ΒΡΧΦ
ΥαΡΤΒΡ÷ ΝΩΖ÷ ΐΘ§ΦΉΓΔ““ΓΔ±ϊ»ΐΈΜΆ§―ßΖ÷±π…ηΦΤΝΥ»γœ¬ Β―ιΖΫΑΗΘΚ
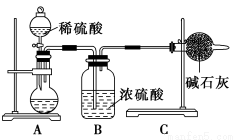
Δώ.ΦΉΆ§―ßΒΡΖΫΑΗ»γΆΦΥυ ΨΘΚ
(1)»γΚΈΦλ―ιAΉΑ÷ΟΒΡΤχΟή–‘ΘΩ___________________________________ΓΘ
(2)ΦΉΆ§―ß÷ΊΗ¥Ϋχ––ΝΥ»ΐ¥Έ Β―ιΘ§ΒΟΒΫΧΦΥαΡΤΒΡ÷ ΝΩΖ÷ ΐΒΡ ΐΨί¥φ‘ΎΫœ¥σΒΡΤΪ≤νΘ§Ρψ»œΈΣΩ…Ρή“ΐΤπ≤βΝΩΫαΙϊΤΪΒΆΒΡ‘≠“ρ «________(Χν–ρΚ≈)ΓΘ
AΘ°ΉΑ÷ΟΡΎ‘≠”–Ω’Τχ÷–ΒΡΕΰ―θΜ·ΧΦΤχΧε“≤±ΜΦν ·Μ“Έϋ ’
BΘ°ΉΑ÷ΟΆβΩ’Τχ÷–ΒΡΥ°’τΤχΚΆΕΰ―θΜ·ΧΦ±ΜΦν ·Μ“Έϋ ’
CΘ°Ζ¥”ΠΆξ≥…ΚσΘ§ΉΑ÷Ο÷–ΒΡΕΰ―θΜ·ΧΦΟΜ”–»Ϊ≤Ω±ΜΦν ·Μ“Έϋ ’
DΘ°Φ”»κœΓΝρΥαΒΡΝΩ≤ΜΉψΓΔΖ¥”Π≤Μ≥δΖ÷
(3)ΈΣΝΥ»ΟΦΉΒΡ Β―ι≤βΝΩΫαΙϊΗϋΉΦ»ΖΘ§‘ΎΤδΥϊ Β―ι≤Ϋ÷ηΕΦ’ΐ»ΖΒΡΧθΦΰœ¬Θ§Ρψ»œΈΣΆΦ÷–ΒΡ Β―ιΉΑ÷Ο”ΠΗΟ»γΚΈΗΡΫχΘΚ___________________________________________ΓΘ
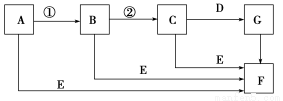
Δρ.““Ά§―ßΒΡΖΫΑΗ «ΘΚ¥”ΆΦ÷–ΥυΧαΙ©ΒΡΉΑ÷Ο÷–―Γ‘ώ Β―ιΉΑ÷ΟΘ§¥ζΧφΦΉΆ§―ß Β―ιΉΑ÷Ο÷–ΒΡBΓΔCΘ§Ά®Ιΐ≤βΕ®Ζ≈≥ωΒΡΕΰ―θΜ·ΧΦΒΡΧεΜΐ(≤ΜΩΦ¬«Εΰ―θΜ·ΧΦ»ή”ΎΥ°)ά¥ΦΤΥψΓΘ
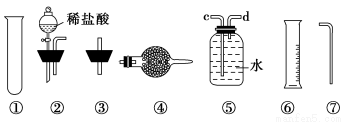
―Γ‘ώΉνΦρΉΑ÷ΟΒΡΝ§Ϋ”Υ≥–ρΈΣ________ΓΘ
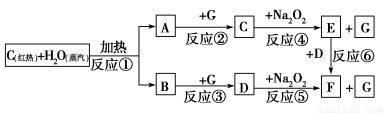
Δσ.±ϊΆ§―ßΒΡΖΫΑΗ «ΘΚ≥Τ»Γ―υΤΖm gΘ§≤Δ»ήΫβΘ§Φ”»κΙΐΝΩ¬»Μ·±Β»ή“ΚΘ§Ιΐ¬ΥΓΔœ¥Β”ΓΔΚφΗ…ΓΔ≥ΤΝΩΘ§ΒΟΙΧΧεn gΓΘ
(1)≈δ÷Τ100 mL 0.10 mol/L BaCl2»ή“ΚΒΡ Β―ι÷–Υυ–ηΒΡ≤ΘΝß“«Τς≥ΐ…’±≠ΓΔ≤ΘΝßΑτΓΔΫΚΆΖΒΈΙήΓΔΝΩΆ≤ΆβΜΙ”–______(Χν“«ΤςΟϊ≥Τ)ΓΘ
(2)ΜλΚœΈο÷–ΧΦΥαΡΤΒΡ÷ ΝΩΖ÷ ΐΈΣ(”ΟmΓΔn±μ Ψ)________ΓΘ
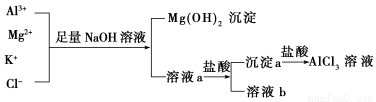
(3)Ca2ΘΪΓΔBa2ΘΪΕΦΩ…“‘ ΙCO32ΓΣ≥ΝΒμΆξ»ΪΘ§ΡήΖώ Ι”Ο¬»Μ·ΗΤ»ή“Κ¥ζΧφ¬»Μ·±Β»ή“ΚΘΩ________(ΧνΓΑΡήΓ±ΜρΓΑΖώΓ±)Θ§‘≠“ρ «ΘΚ______________________________________ΓΘ
Δώ.(1)”Ο÷ΙΥ°Φ–Φ–ΫτAΉΑ÷ΟΒΦΤχΙήΡ©ΕΥΒΡœπΤΛΙήΘ§¥ρΩΣΖ÷“Κ¬©ΕΖ…œ≤ΩΒΡ»ϊΉ”Θ§œρΤδ÷–ΉΔ»κ ΝΩ’τΝσΥ°Θ§¥ρΩΣΜν»ϊΘ§ΩΣ Φ”–…ΌΝΩΥ°ΒΈœ¬Θ§Ιΐ“ΜΜαΕυΘ§Υ°≤ΜΡήΒΈ»κ‘≤ΒΉ…’ΤΩΘ§÷ΛΟςΉΑ÷ΟΤχΟή–‘ΝΦΚΟ(2)CΓΔD(3)‘ΎΉΑΦν ·Μ“ΒΡΗ…‘οΙή”“±Ώ‘ΌΉΑ“ΜΗω Δ”–Φν ·Μ“ΒΡΗ…‘οΙήΘ§Ζά÷ΙΩ’Τχ÷–Υ°Ζ÷ΚΆΕΰ―θΜ·ΧΦ±ΜCΉΑ÷Ο÷–ΒΡΦν ·Μ“Έϋ ’ΓΓΔρ.ΔίΔόΔΏΓΓΔσ.(1)100 mL»ίΝΩΤΩΓΓ(2)106n/197m
(3)ΖώΓΓΙΐΝΩΒΡCa2ΘΪΡή”κOHΘ≠…ζ≥…ΈΔ»ή”ΎΥ°ΒΡ«β―θΜ·ΗΤ≥ΝΒμΕχ”ΑœλΫαΙϊ
ΓΨΫβΈωΓΩΔώ.(1)Φλ―ιAΉΑ÷ΟΤχΟή–‘ΒΡΖΫΖ®ΘΚ”Ο÷ΙΥ°Φ–Φ–ΫτAΉΑ÷ΟΒΦΤχΙήΡ©ΕΥΒΡœπΤΛΙήΘ§¥ρΩΣΖ÷“Κ¬©ΕΖ…œ≤ΩΒΡ»ϊΉ”Θ§œρΤδ÷–ΉΔ»κ ΝΩ’τΝσΥ°Θ§¥ρΩΣΜν»ϊΘ§ΩΣ Φ”–…ΌΝΩΥ°ΒΈœ¬Θ§Ιΐ“ΜΜαΕυΘ§Υ°≤ΜΡήΒΈ»κ‘≤ΒΉ…’ΤΩΘ§÷ΛΟςΉΑ÷ΟΤχΟή–‘ΝΦΚΟΓΘ(2)Β±ΉΑ÷ΟΡΎ‘≠”–Ω’Τχ÷–ΒΡCO2ΤχΧε±ΜΦν ·Μ“Έϋ ’ ±Θ§≤βΒΟCO2ΒΡ÷ ΝΩ‘ω¥σΘ§ΒΦ÷¬≤βΝΩΫαΙϊΤΪΗΏΘΜΒ±ΉΑ÷ΟΆβΩ’Τχ÷–ΒΡΥ°’τΤχΚΆCO2±ΜΦν ·Μ“Έϋ ’ ±Θ§≤βΒΟCO2ΒΡ÷ ΝΩ‘ω¥σΘ§ΒΦ÷¬≤βΝΩΫαΙϊΤΪΗΏΘΜΒ±ΉΑ÷Ο÷–ΒΡΕΰ―θΜ·ΧΦΟΜ”–»Ϊ≤Ω±ΜΦν ·Μ“Έϋ ’ ±Θ§≤βΒΟCO2ΒΡ÷ ΝΩΦθ…ΌΘ§ΒΦ÷¬≤βΝΩΫαΙϊΤΪΒΆΘΜΒ±Φ”»κœΓΝρΥαΒΡΝΩ≤ΜΉψΘ§Ζ¥”Π≤Μ≥δΖ÷ ±Θ§≤ζ…ζΒΡCO2ΝΩ…ΌΘ§≤βΒΟCO2ΒΡ÷ ΝΩΦθ…ΌΘ§ΒΦ÷¬≤βΝΩΫαΙϊΤΪΒΆΓΘ(3)ΈΣΝΥ»ΟΦΉΒΡ Β―ι≤βΝΩΫαΙϊΗϋΉΦ»ΖΘ§”Π‘ΎΉΑΦν ·Μ“ΒΡΗ…‘οΙή”“±Ώ‘ΌΉΑ“ΜΗω Δ”–Φν ·Μ“ΒΡΗ…‘οΙήΘ§Ζά÷ΙΩ’Τχ÷–Υ°Ζ÷ΚΆΕΰ―θΜ·ΧΦ±ΜCΉΑ÷Ο÷–ΒΡΦν ·Μ“Έϋ ’ΓΘ
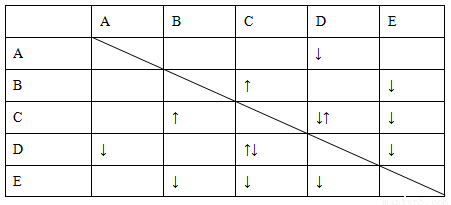
Δρ.”…ΥυΗχΒΡΉΑ÷Ο÷ΣΘ§≤βΕ®CO2ΤχΧεΒΡΧεΜΐ”Π”ΟΓΑ≈≈Υ°ΝΩΤχΖ®Γ±Θ§Υυ“‘―ÔϹΑ÷ΟΔίΓΔΔόΓΔΔΏΘ§ΤχΧε¥”dΩΎΫχΤχΘ§cΩΎ≥ωΥ°Θ§Υ°―ΊΉΑ÷ΟΔΏΫχ»κΉΑ÷ΟΔόΓΘΔσ.(1)≈δ÷Τ“ΜΕ®Έο÷ ΒΡΝΩ≈®Ε»ΒΡ»ή“ΚΘ§”ΟΒΫΒΡ≤ΘΝß“«Τς”–ΘΚ…’±≠ΓΔ≤ΘΝßΑτΓΔ100 mL»ίΝΩΤΩΓΔΫΚΆΖΒΈΙήΓΔΝΩΆ≤ΓΘ(2)n(Na2CO3)ΘΫn(BaCO3)ΘΫ 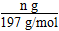 ΘΫ
ΘΫ 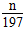 molΘ§‘ρNa2CO3ΒΡ÷ ΝΩΖ÷ ΐΈΣΘΚ
molΘ§‘ρNa2CO3ΒΡ÷ ΝΩΖ÷ ΐΈΣΘΚ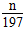 molΓΝ106 g/molΓ¬m gΘΫ106n/197mΓΘ
molΓΝ106 g/molΓ¬m gΘΫ106n/197mΓΘ
(3)”…”ΎΙΐΝΩΒΡCa2ΘΪΡή”κOHΘ≠…ζ≥…ΈΔ»ή”ΎΥ°ΒΡ«β―θΜ·ΗΤ≥ΝΒμΕχ”Αœλ≤βΝΩΫαΙϊΘ§Υυ“‘≤ΜΡή Ι”Ο¬»Μ·ΗΤ»ή“Κ¥ζΧφ¬»Μ·±Β»ή“ΚΓΘ