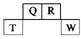
��Ŀ����
��16�֣�W��M��X��Y��Z��Q��ԭ��������������Ķ�����Ԫ�أ�X��Y�ǽ���Ԫ�أ�X����ɫ�ʻ�ɫ������Ԫ�غ˵����֮��Ϊ71��W��Q������������ͬ��Q�ĺ˵������W��2����Z�ĵ��ʺ��������Ϊԭ�Ӿ��塣��ҵ��һ��ͨ�����������ķ������Y�ĵ��ʡ���ش��������⣺
��1��Y�����ӽṹʾ��ͼ_____________;MԪ����Ԫ�����ڱ��е�λ��Ϊ_______________��
��2��д������ʽ����������ָ��������ѧ�������ͣ�W�ĵ���_____________�� ��;
X��W��ԭ�Ӹ�����2:1�γɵĻ����� _____________�� ��.
��3��W��M��X��Y��Z��Qԭ�Ӱ뾶��С�����˳��Ϊ_____________________(��Ԫ�ط��ű�ʾ)��
��4��W��M��Q�γɵ��⻯����ȶ���__________________________(���⻯�����ʽ��ʾ)
ZԪ���⻯��ķ��ӿռ乹��___________________��
��5��X��Y��Q������������Ӧ��ˮ����֮���������ɷ�Ӧ��д����Ӧ�����ӷ���ʽ��
________________________,________________________,______________________;
��6��д��һ����ѧ��Ӧ����ʽ��֤�����н��ۣ�
�ǽ�����M��Wǿ��__________________________________________________________��
�ǽ�����Q��Zǿ��______________________________________________________________��
��1��Y�����ӽṹʾ��ͼ_____________;MԪ����Ԫ�����ڱ��е�λ��Ϊ_______________��
��2��д������ʽ����������ָ��������ѧ�������ͣ�W�ĵ���_____________�� ��;
X��W��ԭ�Ӹ�����2:1�γɵĻ����� _____________�� ��.
��3��W��M��X��Y��Z��Qԭ�Ӱ뾶��С�����˳��Ϊ_____________________(��Ԫ�ط��ű�ʾ)��
��4��W��M��Q�γɵ��⻯����ȶ���__________________________(���⻯�����ʽ��ʾ)
ZԪ���⻯��ķ��ӿռ乹��___________________��
��5��X��Y��Q������������Ӧ��ˮ����֮���������ɷ�Ӧ��д����Ӧ�����ӷ���ʽ��
________________________,________________________,______________________;
��6��д��һ����ѧ��Ӧ����ʽ��֤�����н��ۣ�
�ǽ�����M��Wǿ��__________________________________________________________��
�ǽ�����Q��Zǿ��______________________________________________________________��
��1�� ����2����,�ڢ�A��
����2����,�ڢ�A��
��2��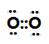 �����ۼ�����
�����ۼ�����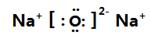 �����Ӽ�����
�����Ӽ�����
��3��r(Na)>r(Al)>r(Si)>r(S)>r(O)>r(F) ��4��HF>H2O>H2S����������
��5��H++OH-=H2O��3H++Al(OH)3=Al3++3H2O��Al(OH)3+OH-=AlO2-+2H2O
��6��2F2+2H2O=4HF+O2 Na2SiO3+H2SO4=Na2SO4+H2SiO3
 ����2����,�ڢ�A��
����2����,�ڢ�A����2��
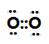 �����ۼ�����
�����ۼ�����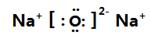 �����Ӽ�����
�����Ӽ�������3��r(Na)>r(Al)>r(Si)>r(S)>r(O)>r(F) ��4��HF>H2O>H2S����������
��5��H++OH-=H2O��3H++Al(OH)3=Al3++3H2O��Al(OH)3+OH-=AlO2-+2H2O
��6��2F2+2H2O=4HF+O2 Na2SiO3+H2SO4=Na2SO4+H2SiO3
X����ɫ�ʻ�ɫ����X��Na�����ʺ��������Ϊԭ�Ӿ�����ǹ裬��Z��Si�����������ķ�����ý���Y�ĵ��ʣ�˵��Y��Al��W��Q������������ͬ�����������ͬһ���壬Q�ĺ˵������W��2������Q��ԭ����������14������W��Qֻ����O��S����������Ԫ�غ˵����֮��Ϊ71���жϳ�M��F��
(2)�������ɷǼ��Լ��γɵĵ��ʣ��������������Ӽ��γɵ����ӻ����
��3��ͬ����Ԫ�����϶���ԭ�Ӱ뾶������ͬ����Ԫ����������ԭ�Ӱ뾶��С��
��4���ǽ�����Խǿ����Ӧ�⻯����ȶ���Խǿ������⻯��ͼ������ƣ�����������ṹ��
��5�������������������������������ǿ�Ҳ���������С�
��6���������õ��ʼ����û�����������������Ӧˮ���������ǿ�����ȽϷǽ�����ǿ����
(2)�������ɷǼ��Լ��γɵĵ��ʣ��������������Ӽ��γɵ����ӻ����
��3��ͬ����Ԫ�����϶���ԭ�Ӱ뾶������ͬ����Ԫ����������ԭ�Ӱ뾶��С��
��4���ǽ�����Խǿ����Ӧ�⻯����ȶ���Խǿ������⻯��ͼ������ƣ�����������ṹ��
��5�������������������������������ǿ�Ҳ���������С�
��6���������õ��ʼ����û�����������������Ӧˮ���������ǿ�����ȽϷǽ�����ǿ����

��ϰ��ϵ�д�
�����Ŀ