��Ŀ����
��ҵ�ϲ�����ͼ��ʾ���װ����ȡ���ý�������������������������ȫ����ʯī�缫��Ӧ����CO��CO2���壬����ڵ�������̼�缫��Ҫ���ϲ��䣮��֪����ܷ�Ӧ����ʽΪ��2Al2O3
4Al+3O2����
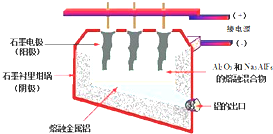
��1��������ӦʽΪ______��������ӦʽΪ______��
��2����������ÿ����agAl��������ʧʯībg���������ϲ���CO��CO2�����ʵ����ֱ���______��
��3���Խ�����Ʒ���п���ʴ���������ӳ���ʹ��������������Ϊ��������H2SO4��Һ�е�⣬���ı����γ�����Ĥ�������缫��ӦʽΪ______��
| ||

��1��������ӦʽΪ______��������ӦʽΪ______��
��2����������ÿ����agAl��������ʧʯībg���������ϲ���CO��CO2�����ʵ����ֱ���______��
��3���Խ�����Ʒ���п���ʴ���������ӳ���ʹ��������������Ϊ��������H2SO4��Һ�е�⣬���ı����γ�����Ĥ�������缫��ӦʽΪ______��
��1�����ݵ�ⷽ��ʽ��֪��������Al���缫����ʽΪ4Al3++12e-=4Al����������O2���缫����ʽΪ6O2--12e-=3O2����
�ʴ�Ϊ��4Al3++12e-=4Al��6O2--12e-=3O2����
��2��n��Al��=
mol����ת�Ƶ���
mol��3=
mol������n��C��=
mol��������xmolCO��ymolCO2��
��
��
��֮��x=
mol��y=
mol��
�ʴ�Ϊ��CO��
mol��CO2��
mol��
��3������Al����������Al2O3���������Һ�����ԣ���������ӦʽΪ2Al+3H2O=Al2O3+6H++6e-��
�ʴ�Ϊ��2Al+3H2O=Al2O3+6H++6e-��
�ʴ�Ϊ��4Al3++12e-=4Al��6O2--12e-=3O2����
��2��n��Al��=
| a |
| 27 |
| a |
| 27 |
| a |
| 9 |
| b |
| 12 |
��
|
��֮��x=
| 3b-a |
| 18 |
| 2a-3b |
| 36 |
�ʴ�Ϊ��CO��
| 3b-a |
| 18 |
| 2a-3b |
| 36 |
��3������Al����������Al2O3���������Һ�����ԣ���������ӦʽΪ2Al+3H2O=Al2O3+6H++6e-��
�ʴ�Ϊ��2Al+3H2O=Al2O3+6H++6e-��

��ϰ��ϵ�д�
�����Ŀ
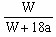 ��100%
��100%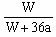 ��100%
��100%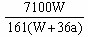 ��100%
��100%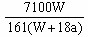 ��100%
��100%