��Ŀ����
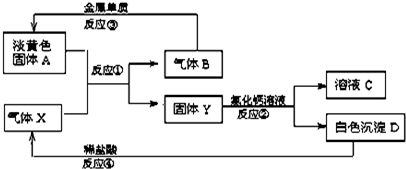
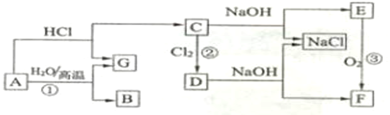
����A��B��C��D��E�������ʣ�����������ת����ϵ������EΪ��ɫ��ĩ������ͼת���о���������
��1��B�����ʵĻ�ѧʽ______��
��2������ͼ1��ʾ��ʵ��װ�ý���C��ˮ�ķ�Ӧ���ش������й����⣺

�ٹ���C��ˮ��Ӧ����D�Ļ�ѧ����ʽ______��
�ڼ��鵼�ܳ�������ķ���Ϊ______��
���ձ��е�����Ϊ______��
��3���ڼ��������£�ij�����ᣨ����A�е�һ��Ԫ�أ���Ũ��Һ��E��Ӧ�����ɵ�������X��Ϊ����X�����ʣ������ͼ2��ʾʵ��װ�ã�
��ʵ������У��۲쵽װ�â��е�Ʒ����ֽ�ĺ�ɫ��ȥ����δ�۲쵽�������Ա仯����һԤ������Ϊ�˴ﵽ��һԤ����������ΪӦ����θĽ���______��
��ʵ�������װ�â��пɹ۲쵽��������______�����μ����ν�ͷ�ι��е��Լ����۲쵽��Һ��Ѫ��ɫ���йص����ӷ���ʽΪ______��______��
��X��һ����Ҫ�Ļ���ԭ�ϣ��û�ѧ����ʽ��ʾX�ڻ��������ϵ�һ����;��______��

��1��B�����ʵĻ�ѧʽ______��
��2������ͼ1��ʾ��ʵ��װ�ý���C��ˮ�ķ�Ӧ���ش������й����⣺

�ٹ���C��ˮ��Ӧ����D�Ļ�ѧ����ʽ______��
�ڼ��鵼�ܳ�������ķ���Ϊ______��
���ձ��е�����Ϊ______��
��3���ڼ��������£�ij�����ᣨ����A�е�һ��Ԫ�أ���Ũ��Һ��E��Ӧ�����ɵ�������X��Ϊ����X�����ʣ������ͼ2��ʾʵ��װ�ã�
��ʵ������У��۲쵽װ�â��е�Ʒ����ֽ�ĺ�ɫ��ȥ����δ�۲쵽�������Ա仯����һԤ������Ϊ�˴ﵽ��һԤ����������ΪӦ����θĽ���______��
��ʵ�������װ�â��пɹ۲쵽��������______�����μ����ν�ͷ�ι��е��Լ����۲쵽��Һ��Ѫ��ɫ���йص����ӷ���ʽΪ______��______��
��X��һ����Ҫ�Ļ���ԭ�ϣ��û�ѧ����ʽ��ʾX�ڻ��������ϵ�һ����;��______��
��1��EΪ��ɫ��ĩ��ͼ��ת���о���������Ӧ����������ء����������ڶ��������������������Ʊ�����������֪����AΪKClO3��BΪH2O2��EΪMnO2��DΪO2���ɣ�2���п�֪������ɫ����C����ˮ��Ӧ��CӦΪNa2O2��
�ʴ�Ϊ��H2O2��
��2���ٹ���������ˮ��Ӧ����������������������Ӧ����ʽΪ��2Na2O2+2H2O=4NaOH+O2����
�ʴ�Ϊ��2Na2O2+2H2O=4NaOH+O2����
�ڷ�Ӧ���ɵ�����Ϊ�������ô����ǵ�ľ������ƿ�ڣ�ľ����ȼ��֤����������
�ʴ�Ϊ���ô����ǵ�ľ������ƿ�ڣ�ľ����ȼ��֤����������
�۷�Ӧ�ų��������ȣ���������������Һ���¶����ߣ��������Ƶ��ܽ�Ƚ��ͣ�����ֻ��ǣ�
�ʴ�Ϊ�����ֻ��ǣ�
��3���ڼ��������£�ij�����ᣨ����A�е�һ��Ԫ�أ���Ũ��Һ��E��Ӧ�����ɵ�������X����������ΪHCl��XΪCl2��
�ټ���װ�â��е�����Ӧ���Ӧ����װ��II����֮�����һ��ʢ��Ũ�����ϴ��ƿ��
�ʴ�Ϊ����װ��II����֮�����һ��ʢ��Ũ�����ϴ��ƿ��
���������������������������ӣ�װ�â��пɹ۲쵽��Һ��dz��ɫ��Ϊ��ɫ���μ��Լ����۲쵽��Һ��Ѫ��ɫ��Ӧ�μ�KSCN����Ӧ����Fe��SCN��3����Ӧ�й����ӷ���ʽΪ��2Fe2++Cl2=2Fe3++2Cl-��Fe3++3SCN-=Fe��SCN��3��
�ʴ�Ϊ����Һ��dz��ɫ��Ϊ��ɫ��2Fe2++Cl2=2Fe3++2Cl-��Fe3++3SCN-=Fe��SCN��3��
���������������Ʊ�Ư�۵ȣ��Ʊ�Ư�۷ɷ���ʽΪ��2Ca��OH��2+2Cl2=CaCl2+Ca��ClO��2+2H2O��
�ʴ�Ϊ��2Ca��OH��2+2Cl2=CaCl2+Ca��ClO��2+2H2O��
�ʴ�Ϊ��H2O2��
��2���ٹ���������ˮ��Ӧ����������������������Ӧ����ʽΪ��2Na2O2+2H2O=4NaOH+O2����
�ʴ�Ϊ��2Na2O2+2H2O=4NaOH+O2����
�ڷ�Ӧ���ɵ�����Ϊ�������ô����ǵ�ľ������ƿ�ڣ�ľ����ȼ��֤����������
�ʴ�Ϊ���ô����ǵ�ľ������ƿ�ڣ�ľ����ȼ��֤����������
�۷�Ӧ�ų��������ȣ���������������Һ���¶����ߣ��������Ƶ��ܽ�Ƚ��ͣ�����ֻ��ǣ�
�ʴ�Ϊ�����ֻ��ǣ�
��3���ڼ��������£�ij�����ᣨ����A�е�һ��Ԫ�أ���Ũ��Һ��E��Ӧ�����ɵ�������X����������ΪHCl��XΪCl2��
�ټ���װ�â��е�����Ӧ���Ӧ����װ��II����֮�����һ��ʢ��Ũ�����ϴ��ƿ��
�ʴ�Ϊ����װ��II����֮�����һ��ʢ��Ũ�����ϴ��ƿ��
���������������������������ӣ�װ�â��пɹ۲쵽��Һ��dz��ɫ��Ϊ��ɫ���μ��Լ����۲쵽��Һ��Ѫ��ɫ��Ӧ�μ�KSCN����Ӧ����Fe��SCN��3����Ӧ�й����ӷ���ʽΪ��2Fe2++Cl2=2Fe3++2Cl-��Fe3++3SCN-=Fe��SCN��3��
�ʴ�Ϊ����Һ��dz��ɫ��Ϊ��ɫ��2Fe2++Cl2=2Fe3++2Cl-��Fe3++3SCN-=Fe��SCN��3��
���������������Ʊ�Ư�۵ȣ��Ʊ�Ư�۷ɷ���ʽΪ��2Ca��OH��2+2Cl2=CaCl2+Ca��ClO��2+2H2O��
�ʴ�Ϊ��2Ca��OH��2+2Cl2=CaCl2+Ca��ClO��2+2H2O��

��ϰ��ϵ�д�
 ѧ���쳵�����ּ��ں�����ҵϵ�д�
ѧ���쳵�����ּ��ں�����ҵϵ�д�
�����Ŀ