��Ŀ����
����Ŀ��ij��ѧʵ��С��Ϊȷ����������ֽ����Ѵ�����������ͼװ�ý���ʵ�顢��Ӧ�������ͷ�Ӧֹͣ��ʱ���������±�:
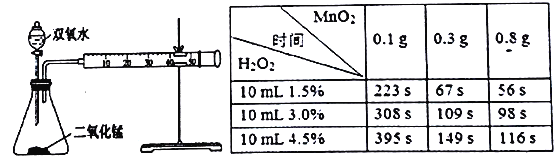
��ش���������:
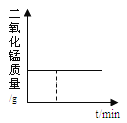
(1)ʢװ˫��ˮ�Ļ�ѧ����������__________��
(2)��μ������װ�õ�������__________��
(3)��ͬŨ�ȵĹ������⣬��ֽ��������Ŷ����������������Ӷ�___________��
(4)��ʵ��Ч���͡���ɫ��ѧ���ĽǶȿ��ǣ�˫��ˮ��Ũ����ͬʱ������_______g�Ķ�������Ϊ�ϼ�ѡ��
(5)ijͬѧ�����������ݺ���Ϊ:��������ͬ�����Ķ�������ʱ,˫��ˮ��Ũ��Խ��,����Ҫ��ʱ���Խ�����䷴Ӧ����Խ�����Ľ��ۣ�����Ϊ�Ƿ���ȷ___________��������_________��(��ʾ:H2O2���ܶȿ���Ϊ�������)��
���𰸡� ��Һ©�� �رշ�Һ©����������ע�����Ļ�˨��������һ�ξ��룬��һ��ʱ����˨�ܹ��ָ���ԭλ�ã���װ�õ������Ժ� �ӿ� 0.3 ����ȷ H2O2��Ũ���������(��1.5%��3.0%)������Ӧ����ʱ��������С�ö�
����������1������ʵ���ҳ������������жϣ�ʢװ˫��ˮ�Ļ�ѧ���������Ƿ�Һ©������2���������װ�õ������Եķ���Ϊ���رշ�Һ©���Ļ�������ע�����Ļ�˨��������һ�ξ��룬��һ��ʱ����˨�ܹ��ָ���ԭλ�ã���װ�õ������Ժã���3���ɱ������ݿ�֪��˫��ˮ������ͬ������Ķ�����������Խ�࣬��Ӧ�����ʱ��Խ�̣�˵����Ӧ����Խ�죻��4�����ݱ������ݣ���˫��ˮ��Ũ����ͬʱ������0.3g�������������0.1g�������̶Ի�ѧ��Ӧ����Ӱ�����ϴ���0.3g�������������0.5g�������̶Է�Ӧ���ʵ�Ӱ�����Ǻܴ����Լ���������̵������Ϊ0.3g����5���ӱ������ݿ�֪����ͬ���3.0%��˫��ˮ�е����ʺ�����1.5%��˫��ˮ�����ʺ����Ķ���������Ӧ��ʱ��ȴ���䷴Ӧʱ��Ķ���С�ö࣬�ɷ�Ӧ���ʼ��㹫ʽ��v=��c/��t���ɵó�����ʵ��������˫��ˮ��Ũ��Խ��ֽ�����Խ�죬�ʣ�����ȷ��H2O2��Ũ�������������1.5%-��3.0%��������Ӧ����ʱ��������С�Ķ���

 ����Ӣ��ϵ�д�
����Ӣ��ϵ�д�����Ŀ�����ǵ���Ҫ�ɷ���CaCO3������������SiO2��MgCO3��ɫ�ص����ʣ��ⶨ�����иƺ����IJ���������ͼ��ʾ:
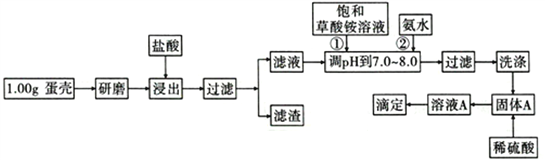
��֪��CaC2O4��MgC2O4��������ˮ��
�ش��������⣺
��1����Ʒ���������õ���������Ҫ��________________________________��
��2���٢ڲ���ʱ�����뱥��(NH4)2C2O4��Һ�Ͱ�ˮ��������_______________________��
��3��ϴ�����ѡ��_____(������ˮ������0. lmol/L�������Һ��)����Ŀ����____________________��
��4����һ���¶��£���2L���ܱ������з��������IJ���ƣ�������ռ������Բ��ƣ�������Ӧ: CaC204(s) ![]() CaO(s)+CO(g)+CO2(g)����ǰ5min ������ CaO ������Ϊ11.2g����ö�ʱ����v(CO)=_______��
CaO(s)+CO(g)+CO2(g)����ǰ5min ������ CaO ������Ϊ11.2g����ö�ʱ����v(CO)=_______��
��5���ζ��������ñ����Ը��������Һ�ζ����ɵIJ��ᣬͨ���������Ķ�����ϵ���ɼ������Ƶĺ����� ����1:����ҺA������ˮϡ����250mL��
����2:ȡϡ�ͺ����Һ25.00mL����ƿ�У���ϡH2SO4�ữ��
����3:��0.0190 mol��L-1KMnO4��Һ�ζ�����2������Һ���յ㣬����KMnO4��ҺV1mL��
����4���ظ�����2������3�IJ���3�Σ���¼�������±���
ʵ���� | KMnO4��Һ��Ũ�ȣ�mol /L) | KMnO4Һ����������mL) |
1 | 0.0190 | V1=20.02 |
2 | 0.0190 | V2= 20.00 |
3 | 0.0190 | V3=19.98 |
4 | 0.0190 | V4 = 20.80 |
��KMnO4��Һ�Ͳ�����Һ��ϡ�����з�Ӧ�����ӷ���ʽΪ____________________��
�ڵζ��յ��������________________________________________��
�۸õ�����CaCO3����������=_______%����˷���õĸƺ���_________ʵ��ֵ(����>����=������<��)
����Ŀ�����г��ӷ���ѡ�ô������
����(������Ϊ����) | ���ӷ��� | |
A | ������(��) | ���� |
B | ��ϩ(SO2) | NaOH��Һ��ϴ�� |
C | ����(��ϩ) | ��ˮ����Һ |
D | ������Һ(NaCl) | ���� |
A.AB.BC.CD.D