��Ŀ����
����Ŀ����֪��2H2S��g��+O2��g�� = S2��s��+2H2O��l�� ��H= -632 kJmol-1����ͼΪ����ĤH2S ȼ�ϵ�ص�ʾ��ͼ������˵����ȷ����

A.��״���£�ÿ 11.2 LH2S ���뷴Ӧ����1 mol H+����������Ĥ����������
B.��ع���ʱ�����Ӵӵ缫b ��������Ĥ����缫 a
C.��·��ÿ���� 4 mol ���ӣ�����ڲ��ͷ�632 kJ ������
D.�缫b �Ϸ����ĵ缫��ӦʽΪ��O2+4e-+2H2O = 4OH-
���𰸡�A
��������
�ɻ�ѧ����ʽ![]() ��֪O2������ԭ��ӦΪ�������缫��ӦΪ��
��֪O2������ԭ��ӦΪ�������缫��ӦΪ��![]() ��H2S����������Ӧ��Ϊ��Դ�������缫��ӦΪ��
��H2S����������Ӧ��Ϊ��Դ�������缫��ӦΪ��![]() ���Դ˷�����
���Դ˷�����
A. ��״���£�ÿ 11.2 LH2S ���뷴Ӧ����H2S���ʵ���Ϊ0.5mol���ɵ缫����ʽ![]() ��֪����1 mol H+����������Ĥ��������������A��ȷ��
��֪����1 mol H+����������Ĥ��������������A��ȷ��
B. ��ع���ʱ�����ӴӸ������ⲿ��·�������������Ӿ��缫a������������缫 b����B����
C. ��Ӧ�ɻ�ѧ��ת��Ϊ���ܣ����ͷŵ�����С��632 kJ����C����
D. �缫b �Ϸ����ĵ缫��ӦʽΪ��![]() ����D����
����D����
�ʴ�ѡ��A��

 �����Ļ���������人������ϵ�д�
�����Ļ���������人������ϵ�д� ���������ּ���ÿһ��ȫ�º�����ҵ��ϵ�д�
���������ּ���ÿһ��ȫ�º�����ҵ��ϵ�д� ��ٽ������½������������ϵ�д�
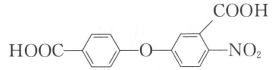
��ٽ������½������������ϵ�д�����Ŀ��![]() ��Ҫ����ѡ�����αװͿ�ϵ����ϵȡ����ܿ�ʯ
��Ҫ����ѡ�����αװͿ�ϵ����ϵȡ����ܿ�ʯ![]() ��
��![]() ��CoO������
��CoO������![]() ��
��![]() ��
��![]() ��
��![]() ����
����![]() ��������ͼ1��
��������ͼ1��

�±��г��˼������������������������pH![]() ��������ȫ����ָ��Һ������Ũ�ȵ���
��������ȫ����ָ��Һ������Ũ�ȵ���![]()
|
|
|
|
| |
��ʼ������pH |
|
|
|
|
|
������ȫ��pH |
|
|
|
|
|
![]() д������ȡ������
д������ȡ������![]() ������Ӧ�����ӷ���ʽ______��
������Ӧ�����ӷ���ʽ______��
![]() ����ȡ�������
����ȡ�������![]() �⣬�ܿ�ʯ�л��ܱ�
�⣬�ܿ�ʯ�л��ܱ�![]() ��ԭ��������______��
��ԭ��������______��
![]() ��
��![]() ��Ŀ��������
��Ŀ��������![]() ��
��![]() �������������ɵIJ��������______��
�������������ɵIJ��������______��
![]() ��������������
��������������![]() ��Һ����
��Һ����![]() ��Һ��Ҫ
��Һ��Ҫ![]() �����½��У����˵ļ��ȷ�ʽΪ______���¶ȿ�����
�����½��У����˵ļ��ȷ�ʽΪ______���¶ȿ�����![]() ��ԭ��Ϊ______��
��ԭ��Ϊ______��
![]()
![]() ��ʵ�鷽�������������������Һ�м���______������
��ʵ�鷽�������������������Һ�м���______������![]() ��Һ���ܵ�
��Һ���ܵ�![]() ʵ������ʹ���Լ���
ʵ������ʹ���Լ���![]() ��Һ��������ȡ��������
��Һ��������ȡ��������![]() ��
��
����Ŀ���ش��������⣺
(1)�����(�۵�1410 ��)�����õİ뵼����ϡ��ɴֹ��ƴ���������£�
![]()
��������SiCl4(g)�ƴ���ķ�Ӧ�У����ÿ����1.12 kg����������a kJ������д���÷�Ӧ���Ȼ�ѧ����ʽ��___��
(2)����ˮú���ϳɶ����ѵ��ܷ�ӦΪ��3CO(g)��3H2(g) = CH3OCH3(g)��CO2(g) ��H��-246.4 kJ��mol-1�������Է�Ϊ��������Ӧ�ֱ����£�2CO(g)��4H2(g) = CH3OCH3(g)��H2O(g) ��H1����205.1 kJ��mol-1��CO(g)��H2O(g) = CO2(g)��H2(g)����H2��_____����֪CH3OCH3(g)��ȼ����Ϊ1455 kJ��mol��1��д����ʾ��ȼ���ȵ��Ȼ�ѧ����ʽ��__________��
(3)��O2��HClת��ΪCl2�������Ч�棬������Ⱦ��һ�������²�÷�Ӧ������ c(Cl2)���������£�
t/min | 0 | 2.0 | 4.0 | 6.0 | 8.0 |
c(Cl2)/10-3 mol/L | 0 | 1.8 | 3.7 | 5.4 | 7.2 |
����2.0��6.0 min��HCl�ķ�Ӧ����Ϊ_____��
(4)��һ���¶��µĶ��������У����������������ٷ����仯ʱ������������ѹǿ�������������ܶȣ����������������ʵ���������������ƽ����Է���������������������ɫ����c(I2):c(H2):c(HI)=1:1:2���������İٷֺ�������˵��I2(g)��H2(g) ![]() 2HI(g)�ﵽƽ��״̬����____��
2HI(g)�ﵽƽ��״̬����____��
(5)�ں��º�ѹ��������ʱ����ӦA��B = C��Dһ�����Է����е���������H__0����S___0(�<������>����=��)��