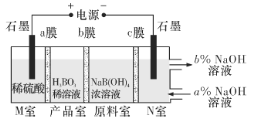
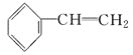
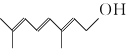
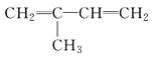��Ŀ����
����Ŀ������һ����Ȼ��ֲ��Ϲ��Ԫ�أ��ںܶ�����ж����и�Ԫ�أ��ش��������⣺
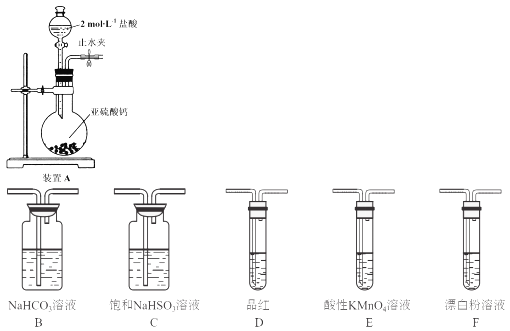
(1)װ��A�з�Ӧ�Ļ�ѧ����ʽΪ___________��
(2)ʹ��Һ©����Һ��˳�����µIJ�����___________��
(3)ѡ�������װ�ú�ҩƷ̽��������������������ǿ����
�ټ�ͬѧ��Ϊ����A��C��F��˳������װ�ü���֤������ͬѧ��Ϊ�÷�������������������___________��
�ڱ�ͬѧ��Ƶĺ���ʵ�鷽��Ϊ��A��C��_____��___��D��F������װ��C��������_____��֤�������������ǿ�ڴ������ʵ��������____��
(4)K2S2O3����ǿ�����ԣ���ͨ�����H2SO4��K2SO4�Ļ����Һ�Ƶã���������ӦʽΪ_____����ȡ0.2500g��Ʒ�ڵ���ƿ�У���100mLˮ�ܽ⣬�ټ���8.000g KI����(�Թ���)����ʹ���ַ�Ӧ����������������Һ�ữ���Ե���Ϊָʾ������cmol/L��Na2S2O3��Һ�ζ����յ㣬����Na2S2O3��ҺVmL������Ʒ��K2S2O8�Ĵ���Ϊ______%(�ú�c��V�Ĵ���ʽ��ʾ����֪��S2O82��+2I��=2SO42��+I2��2S2O32��+I2=S4O62��+2I��)��
���𰸡�CaSO3+2HCl=CaCl2+SO2��+H2O ���·�Һ©������������(��ʹ��Һ©��ƿ���ϵİ��۶�ƿ����С��)��Ȼ������ת�²����� SO2ͨ����������Һ����������ԭ��Ӧ������֤��ǿ���Ʊ������ԭ�� B E ��ȥHCl���� D��Ʒ�첻��ɫ��F�г��ְ�ɫ���� 2SO42����2e�� =S2O82�� 54cV
��������
��1���������������ᷴӦ�����ɶ�������
��2���ӷ�Һ©�������淶���
��3��SO2ͨ����������Һ����������ԭ��Ӧ������֤��ǿ���Ʊ������ԭ����Ӧ����֤���������Ա�̼��ǿ������֤̼�������ǿ�ڴ����ᣬ�Ӷ�˵�������������ǿ�ڴ����
��4�������÷�Ӧ��֮�������ϵ���㡣
��1��������������Ʒ�Ӧ�����Ȼ��ơ�����������ˮ����Ӧ����ʽΪ��CaSO3+2HCl=CaCl2+SO2��+H2O���ʴ�Ϊ��CaSO3+2HCl=CaCl2+SO2��+H2O��
��2����Һ©��ʹ��ʱӦʹ����ѹǿƽ�⣬������Һ˳�����£���ҺʱӦ���·�Һ©������������(��ʹ��Һ©��ƿ���ϵİ��۶�ƿ����С��)��Ȼ������ת�²��������ʴ�Ϊ�����·�Һ©������������(��ʹ��Һ©��ƿ���ϵİ��۶�ƿ����С��)��Ȼ������ת�²�������
��3���ٴ��������ǿ�����ԣ�����������л�ԭ�ԣ����߷���������ԭ��Ӧ�����ܷ������ֽⷴӦ������̽��������������������ǿ�����ʴ�Ϊ��SO2ͨ����������Һ����������ԭ��Ӧ������֤��ǿ���Ʊ������ԭ����
��װ��A��������������Ʒ�Ӧ�Ʊ������������������ӷ����ƵõĶ��������л���HCl��Ӧ�ñ��͵����������Ƴ�ȥHCl����ͨ��̼��������Һ������������̼��������Һ��Ӧ���ɶ�����̼��������֤���������Ա�̼��ǿ�������Ը��������Һ������ȥ������̼�еĶ���������Ʒ����Һ���������̼�ж��������Ƿ�������ٽ�������̼ͨ��F�У���Ӧ���ɴ����ᣬ��֤̼�������ǿ�ڴ����ᣬ�Ӷ�˵�������������ǿ�ڴ����ᣬ��װ������˳��ΪA��C��B��E��D��F������װ��C�������dz�ȥHCl���壬D��Ʒ�첻��ɫ��F�г��ְ�ɫ��������֤�������������ǿ�ڴ����ᣬ�ʴ�Ϊ��B��E����ȥHCl��D��Ʒ�첻��ɫ��F�г��ְ�ɫ������
��4���������֪��SO42��������ʧ���ӷ���������Ӧ����S2O82�����缫��ӦʽΪ2SO42����2e�� =S2O82������������ӷ���ʽ�ɵù�ϵʽS2O82-��2S2O32-��n��S2O32-��=cV��10��3mol����n��S2O82-��=![]() cV��10��3mol����Ʒ��K2S2O8�Ĵ���Ϊ
cV��10��3mol����Ʒ��K2S2O8�Ĵ���Ϊ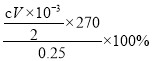 =54cV%���ʴ�Ϊ��2SO42����2e�� =S2O82����54cV��
=54cV%���ʴ�Ϊ��2SO42����2e�� =S2O82����54cV��

 ��ĩ1�����ʽ���������ϵ�д�
��ĩ1�����ʽ���������ϵ�д�����Ŀ��������ʵ�����������ʵ��������������������һ����
ѡ�� | �Թ�ʵ����������� | ʵ����� |
A | ��KI��������Һ�еμ���ˮ����Һ�����ɫ | �����ԣ�I2��Cl2 |
B | �������еμ�Ũ���ᣬ���DZ�� | Ũ��������ˮ�� |
C | ��ij��Һ�м��������������ټ���BaCl2��Һ���а�ɫ�������� | ��Һ�к��� |
D | ��ij����Һ�м���Ũ�������Ʋ����ȣ���ʪ��ĺ�ɫʯ����ֽ�����Թܿڣ���ֽ���� | ��Һ�к��� |
A.AB.BC.CD.D
����Ŀ��I��K2Cr2O7�����ڼ��˾���Ƿ�ƺ��ʻ��Cr2O72��(��ɫ)+CH3CH2OH��Cr3+(��ɫ)+CH3COOH(δ��ƽ)
(1)��̬Crԭ�ӵļ۵��ӹ������ʽΪ_________��
(2)�ʰ���Ľṹ��ʽΪNH2CH2COOH���÷���������Ԫ�صĵ�һ�������ɴ�С��˳��Ϊ___________��̼ԭ�ӵĹ���ӻ�����Ϊ_______��
(3)��֪Cr3+�ȹ���Ԫ��ˮ�����ӵ���ɫ���±���ʾ��
���� | Sr3+ | Cr3+ | Fe2+ | Zn2+ |
ˮ�����ӵ���ɫ | ��ɫ | ��ɫ | dz��ɫ | ��ɫ |
�����ԭ�ӽṹ�Ʋ�Sc3+��Zn2+��ˮ������Ϊ��ɫ��ԭ��Ϊ___________��
��ZnCl2Ũ��Һ�����ڳ�ȥ������������������FeO��Ӧ�ɵ� Fe[Zn(OH)Cl2]2��Һ��
(4) Fe[Zn(OH)Cl2]2��ˮ��Һ�в����ڵ�������������________(��ѡ����ĸ)��
A ���Ӽ� B ���ۼ� C ������ D ��λ�� E ���»��� F ���
��ij�������ģ������ͼ��ʾ��
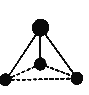
(5)��֪����1��̼ԭ�Ӻ�3����ԭ�ӣ���д�������Ļ�ѧʽ��_____��
����ͭ����Ԫ���γɵľ�������ͼ��ʾ��
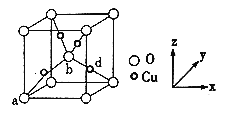
(6)������Cu���ȵط�ɢ���������ڲ���a��b�������������Ϊ(0��0��0)��(1/2��1/2��1/2)����d���������Ϊ___________����֪�þ�����ܶ�Ϊ��g/cm3��NA�ǰ����ӵ�����ֵ��������Ϊ___________cm(�г�����ʽ����)��