��Ŀ����
����Ŀ�����������з����˷ܼ�����ʧ��ƽ��Ҳ�ܻ����������¡�ij���˷ܼ��Ľṹ��ʽ��ͼ��ʾ���йظ����ʵ�˵������ȷ����(����)
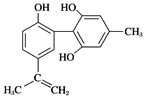
A. ��������FeCl3��Һ����ɫ�������ڱ���ͬϵ��
B. ��������KMnO4��Һ����ɫ��ȥ����֤����ṹ�д���̼̼˫��
C. 1 mol�����ʷֱ���Ũ��ˮ��H2��Ӧʱ�������Br2��H2�ֱ�Ϊ4 mol��7 mol
D. �÷���������̼ԭ��һ����ƽ��
���𰸡�C
��������
A������ͬϵ����ֻ��1�������������ʺ�2�����������DZ���ͬϵ�������-OH����FeCl3��Һ����ɫ��ѡ��A����B��̼̼˫����̼̼��������-OH����-OH�Ⱦ��ܱ���������������KMnO4��Һ����ɫ��ȥ��������֤����ṹ��̼̼˫�������ǻ����ֻ������ٴ���һ�֣�ѡ��B����C����-OH���ڶ�λ����ˮ����ȡ����̼̼˫������ˮ�����ӳɣ���1mol��������Ũ��ˮ��Ӧ��������Ϊ4mol��������̼̼˫���������������ӳɣ���1mol��������������Ӧʱ����������Ϊ7mol��ѡ��C��ȷ��D��������̼̼˫����Ϊƽ��ṹ����ֱ��������ԭ����ͬһƽ���ڣ���÷����е�����̼ԭ�ӿ��ܹ�ƽ�棬ѡ��D����ѡC��

 ȫ�ŵ�����Ԫ�ƻ�ϵ�д�
ȫ�ŵ�����Ԫ�ƻ�ϵ�д�����Ŀ�����ǻ�ѧʵ���Ҽ����������е���Ҫ���ʣ�Ӧ�ù㷺��
��1����֪25��ʱ��N2(g)��O2(g)![]() 2NO(g) ��H=+183 kJ/mol
2NO(g) ��H=+183 kJ/mol
2H2(g)��O2(g)��2H2O(l) ��H=��571.6 kJ/mol
4NH3(g)��5O2(g)��4NO(g)��6H2O(l) ��H=��1164.4 kJ/mol
��N2(g)��3H2(g)![]() 2NH3(g) ��H=______kJ/mol
2NH3(g) ��H=______kJ/mol
��2���ں��º����ܱ������н��кϳɰ���Ӧ����ʼͶ��ʱ������Ũ�����±���
N2 | H2 | NH3 | |
Ͷ�Ϣ� | 1.0 mol/L | 3.0 mol /L | 0 |
Ͷ�Ϣ� | 0.5 mol/L | 1.5 mol/L | 1.0 mol/L |
�ٰ�Ͷ�Ϣ���з�Ӧ����ôﵽ��ѧƽ��״̬ʱH2��ת����Ϊ40%������¶��ºϳɰ���Ӧ��ƽ�ⳣ������ʽΪ_______��
�ڰ�Ͷ�Ϣ���з�Ӧ����ʼʱ��Ӧ���еķ���Ϊ________�������������
���������¶ȣ���ϳɰ���Ӧ�Ļ�ѧƽ�ⳣ��________����������С�����䡱����
��L��L1��L2����X�ɷֱ����ѹǿ���¶ȡ���ͼ��ʾLһ��ʱ���ϳɰ���Ӧ��H2(g)��ƽ��ת������X�ı仯��ϵ��

�� X��������������______��
�� �ж�L1��L2�Ĵ�С��ϵ������������______��
��3���绯ѧ���������������ڼ�����NH3�ĺ������乤��ԭ��ʾ��ͼ���£�
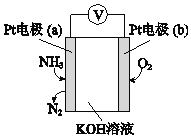
�ٵ缫b�Ϸ�������______��Ӧ�����������ԭ������
��д���缫a�ĵ缫��Ӧʽ_________��