��Ŀ����
6���о����ʵ����ʣ��������ù۲졢ʵ�顢����ͱȽϵȷ�������1���������ʵ���ɺ����ʿɶ����ʽ��з��࣮��Na��K��H��O��C��S��N���������ֻ�����Ԫ�����һ�ֺ��ʵ����ʣ��ֱ������±��У�
| ������� | �� | �� | �� | ������ |
| ��ѧʽ | ��H2S��H2SO4��HNO3��H2SO3��HNO2��H2CO3 | ��KOH��NaOH | ��NH4NO3 | ��CO2 ��Na2O2 |
��2����ˮ�к����ȡ��塢�⡢þ��Ԫ�أ��������·�Ӧ��Cl2+2Br-=2Cl-+Br2��Br2+2I-=2Br-+I2��
�ɴ˿��жϣ�Cl-��Br-��I-��ԭ����ǿ������˳����I-��Br-��Cl-��
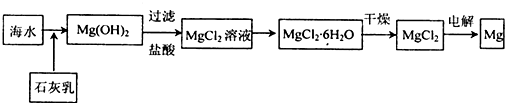
���� ��1�������ᡢ��μ�������ĸ���д����Na��K��H��O��C��S��N���������ֻ�����Ԫ����ɺ��ʵij������ʣ��������ˮ��Һ�л�����״̬�µ���Ļ��������������â���ΪO2��Դ�ǹ������ƺ�ˮ��������̼��Ӧ����������
��2���������������Դ���������������жϣ�
��� �⣺��1����Na��K��H��O��C��S��N����Ԫ���е����ֻ�������Ԫ�أ���ɵ��������ᡢ���ᡢ�����ᡢ�����ᡢ������ȣ���ɵļ����������ơ��������أ���ɵ��������ᱵ�����ᱵ������صȣ���ɵ���������ˮ������������������������ء������ơ�̼���ơ�̼��صȣ��������ˮ��Һ�л�����״̬�µ���Ļ�����ᡢ��Ρ����ý��������ﶼ�ǵ���ʣ�������̼�����������ﱾ�����ܵ���������Ƿǵ���ʣ�����������â���ΪO2��Դ�ǹ������ƺ�ˮ��������̼��Ӧ����������
�ʴ�Ϊ��H2S��H2SO4��HNO3��H2SO3��HNO2��H2CO3�ȣ�KOH��NaOH�ȣ��ܣ�2Na2O2+2CO2=2Na2CO3+O2��2Na2O2+2H2O=4NaOH+O2��
��2��Cl2+2Br-=2Cl-+Br2��Br2+2I-=2Br-+I2���������������Դ�����������ɴ˿��жϣ�Cl-��Br-��I-��ԭ����ǿ������˳����I-��Br-��Cl-��
�ʴ�Ϊ��I-��Br-��Cl-��
���� ���⿼����������ԭ��Ӧ��������������ǿ����������������ʡ��ǵ���ʸ��������Ӧ�ã����ʷ��������ǽ���ؼ�����Ŀ�Ѷ��еȣ�

| A�� | FeBr2��Һ��ͨ��������Cl2��Cl2+2Fe2+�T2Fe3++2Cl- | |
| B�� | ����ʯ���ڴ��CaCO3+2H+�TCa2++CO2��+H2O | |
| C�� | ��NaHSO4��Һ�еμ�Ba��OH��2���պó�����ȫ��2H++SO42-+Ba2++2OH-�TBaSO4��+2H2O | |
| D�� | NaOH��Һ��ͨ�����SO2���壺SO2+2OH-�TSO32-+H2O |
| A�� | ������ˮ�ࡢ�մɾ��������ǽ������� | |
| B�� | ������ˮ�ࡢ�մɶ����ɴ��ʯ��ʯ��ʯӢ�Ƶ� | |
| C�� | �ߴ��ȵĶ�������㷺�����������ά | |
| D�� | �뵼����Ͼ������ö�����������ȡ |
| A�� | ϡ����������������Һ��ϣ�SO42-+Ba2+�TBaSO4�� | |
| B�� | ��������ͭ��Һ��Ӧ��2Na+2Cu2+�T2Na++Cu | |
| C�� | Cl2ͨ��NaOH��Һ��Cl2+2OH-�TCl-+CLO-+H2O | |
| D�� | ����ͭ�������O2-+2H+�TH2O |
| A�� | 3mL��2mol•L-1������ | B�� | 4mL��6mol•L-1������ | ||
| C�� | 2mL��4mol•L-1������ | D�� | 5mL��18mol•L-1������ |
| A�� | ����ˮ���Al��OH��3����������ˮ�������������ˮ�ľ��� | |
| B�� | �ں������������п�飬�ɼ�������ĸ�ʴ���� | |
| C�� | ͨ���ع��ͣ���֬���ļ���ˮ�����Ʒ��� | |
| D�� | ���MgCl2������Һ�����Ƶý���þ |
| A�� | ���嵥����һ�����ڦҼ������ܴ��ڦм� | |
| B�� | �������м۵����Ų�ʽ�Ļ�̬ԭ���У���3s23p1����3s23p2����3s23p3����3s23p4����һ�����������Ǣ� | |
| C�� | NH4+��CH4���ڵȵ����壬���幹�Ͷ����������� | |
| D�� | BF3��NCl3��H2O��XeF4��BeCl2����������ԭ�Ӷ����������Ϊ8���ӽṹ����NCl3 |
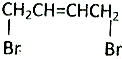 �ɾ��IJ���Ӧ��ȡHOOCCH=CHCOOH��
�ɾ��IJ���Ӧ��ȡHOOCCH=CHCOOH��