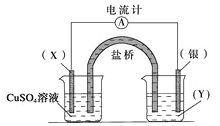
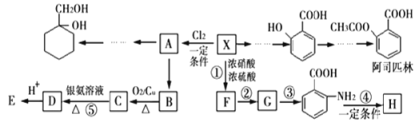
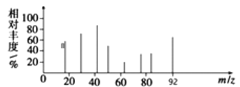
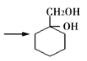
��Ŀ����
����Ŀ����ҵ�ϣ�������������ԭ��������β���е�CO��NO��
����1�����������������������Ϊ��Ӧ��2CO��g����O2��g��![]() 2CO2��g���Ĵ�����ͼ�ױ�ʾ����ͬ�ĺ����ܱ���������ͬ��ʼŨ�ȡ���ͬ��Ӧʱ����£�ʹ��ͬ�����IJ�ͬ��������������͡����ͣ���ʱ��CO��ת�������¶ȵĹ�ϵ��
2CO2��g���Ĵ�����ͼ�ױ�ʾ����ͬ�ĺ����ܱ���������ͬ��ʼŨ�ȡ���ͬ��Ӧʱ����£�ʹ��ͬ�����IJ�ͬ��������������͡����ͣ���ʱ��CO��ת�������¶ȵĹ�ϵ��
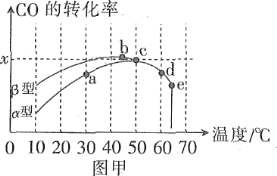
��1����a��b��c��d�ĵ��У�δ�ﵽƽ��״̬����____��
��2����֪c��ʱ������O2Ũ��Ϊ0.04mol��L-1����50��ʱ�������������������COת����Ӧ��ƽ�ⳣ��K��____���ú�x�Ĵ���ʽ��ʾ����
��3�����й���ͼ��˵����ȷ����____��
A��COת����Ӧ��ƽ�ⳣ��K��a����K��c��
B���ھ�δ�ﵽƽ��״̬ʱ��ͬ�������������������COת�����ʱ�����Ҫ��
C��b��ʱCO��O2����֮�䷢����Ч��ײ�ļ���������ʵ����������
D��e��ת���ʳ���ͻ���ԭ��������¶����ߺ����ʧȥ����
����2����ԭ����ij���ܴ������Դ��������ͳ�β���е�̼�̣�C����NOx����ͬ�¶��£���ģ��β�����ɷ������ʾ������ͬ������ͨ���ô�����������в��CO2��N2��N2O����NO��������ݽ����ͼ����ʾ��
ģ��β�� | ���壨10 mol�� | ̼�� | ||
NO | O2 | He | ||
���ʵ�����mol�� | 0.025 | 0.5 | 9.475 | n |
��4��375��ʱ������ų��������к�0.45molO2��0.052molCO2����Y�Ļ�ѧʽΪ____��
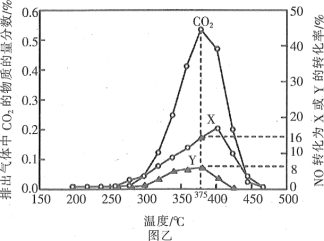
��5��ʵ������в���NOģ��NOx����������NO2��ԭ����____��
���𰸡�a ![]() BD N2 ���ڴ��ڷ�Ӧ2NO2
BD N2 ���ڴ��ڷ�Ӧ2NO2![]() N2O4�ᵼ��һ���ķ������
N2O4�ᵼ��һ���ķ������
��������
����Ϊ��ѧƽ����ۺ��⣬���ж��м��㣬ƽ����жϺ�ƽ�ⳣ���ļ��㡣ͼ��Ҫ���ݺ����������������ߵı仯��������жϡ�
��1��CO��O2��Ӧ�Ƿ��ȷ�Ӧ�����ﵽƽ��������¶ȣ�CO��ת���ʽ��ͣ����ԣ�b��c��d���ʾƽ��״̬��a���Ӧ��״̬�Dz�ƽ��״̬��
��2����CO��ʼŨ��Ϊa mol��L-1��
2CO��g����O2��g��![]() 2CO2��g��
2CO2��g��
��ʼŨ�ȣ�mol��L-1���� a 0
ת��Ũ�ȣ�mol��L-1���� ax ax
ƽ��Ũ�ȣ�mol��L-1����a��1-x�� 0.04 ax
![]() ��
��
��3��CO��O2��Ӧ�Ƿ��ȷ�Ӧ��a��δ�ﵽƽ�⣬û��ƽ�ⳣ�������ﵽƽ����¶����ߣ�ƽ�������ƶ���ƽ�ⳣ��K��С��A����۲�ͼ��֪��������������CO��ת�����ʴ�����������B����ȷ����Ч��ײ�����뷴Ӧ�����йأ��¶�Խ�ߣ���Ӧ����Խ����Ч��ײ����Խ�ߣ�����ͼ����e����Ч��ײ������ߣ�C���������Ҫһ�������¶ȣ�ת���ʳ���ͻ�䣬���������¶ȸ߶�����ʧȥ���ԣ�D����ȷ����ѡBD��
��4���۲�ͼ��NO����X��Y��ת����֮��Ϊ24������NO�μӷ�Ӧ�����ʵ���n��NO����0.025mol����16����8������0.006 mol������ԭ���غ㣬X��Y�����ʵ���֮�͵���0.003mol���ȸ���Oԭ���غ���N2O�����ʵ������ٸ���N�غ���N2�����ʵ�����
��Ͷ������ʺ��ų������ʵ����ʵ���֪��
NO��C��O2��CO2��N2��N2O
���ʵ�����0.006 0.05 0.052 0.001 0.002
����ͼ��X�������Y��2������YΪN2��
��5��NO2��N2O4���棬���ߴ���ת��ƽ�⣬��NO2ģ��ʵ�������ϴ���

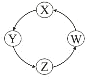
����Ŀ�����и��������У��������ͼʾ������һ�������£�һ��ת����ϵ������У� ��
��� | X | Y | Z | W |
|
�� | Si | Na2SiO3 | H2SiO3 | SiO2 | |
�� | Na | NaOH | Na2CO3 | NaCl | |
�� | Cl2 | Ca(ClO)2 | HClO | HCl | |
�� | Fe | FeCl3 | FeCl2 | Fe(OH)2 |
A.�٢ڢ�B.�٢�C.�ڢ�D.�٢�