��Ŀ����
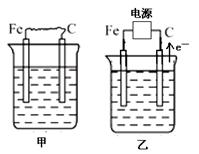
A��B��C����ǿ����ʣ�������ˮ�е����������ΪNa+ ��Ag+��NO3-��SO42-�� Cl-������ͼ��ʾװ���У��ס��ҡ��������ձ����ηֱ�ʢ��������A�� B�� C������Һ���缫��Ϊʯī�缫����ͨ��Դ������һ��ʱ�������ձ���c�缫����������10.8�ˡ������¸��ձ�����Һ��pH����ʱ��t�Ĺ�ϵ��ͼ��ʾ���ݴ˻ش��������⣺

��1��MΪ��Դ��_______������д���������������ס��������ձ��еĵ���ʷֱ�Ϊ_______��_______����д��ѧʽ����
��2������缫f�����ɵ�����Ϊ_______mol��
��3��д�����ձ��еĵ�ⷴӦ����ʽ�� ________________________________��
��4�����������Һ�����Ϊ10L�������Һ��pHΪ___________ ��
��5��Ҫʹ���ָ���ԭ����״̬��Ӧ���������� ����д��ѧʽ����

��1��MΪ��Դ��_______������д���������������ס��������ձ��еĵ���ʷֱ�Ϊ_______��_______����д��ѧʽ����
��2������缫f�����ɵ�����Ϊ_______mol��
��3��д�����ձ��еĵ�ⷴӦ����ʽ�� ________________________________��
��4�����������Һ�����Ϊ10L�������Һ��pHΪ___________ ��
��5��Ҫʹ���ָ���ԭ����״̬��Ӧ���������� ����д��ѧʽ����
��1���� ��1�֣� ��NaCl��1�֣� �� AgNO3��1�֣�
��2��0.025mol��2�֣�
��3��4AgNO3+2H2O = 4Ag+O2+4HNO3��3�֣�
��4��12 ��2�֣�
��5�� H2O ��2�֣�
��2��0.025mol��2�֣�
��3��4AgNO3+2H2O = 4Ag+O2+4HNO3��3�֣�
��4��12 ��2�֣�
��5�� H2O ��2�֣�
��

��ϰ��ϵ�д�
�����Ŀ
 CaCl2 + 2NH3�� + 2H2O
CaCl2 + 2NH3�� + 2H2O
 2CO2(g)��3H2O(l) ����H��0
2CO2(g)��3H2O(l) ����H��0 �����ڴ˱���ԭ
�����ڴ˱���ԭ