��Ŀ����
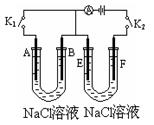
(16��) ��ͼ�мס��ҡ����ĵ缫���϶���ʯī���������б����ȼҵ����ʾ��ͼ��
��1�����ס������ձ���ʢ��CuSO4��Һ��
�ټ��������ϵĵ缫��ӦʽΪ_______________________________________��
����װ�ù���һ��ʱ������ձ��м��������ļ�ʽ̼��ͭ��Cu2(OH)2CO3������ʹ��Һ�ָ�����ʼ״̬����д�����ʱ������װ�÷��������з�Ӧ�Ļ�ѧ����ʽ
________________________________________________________________________��
��2�����ס������ձ���ʢ�ű���NaCl��Һ��
�ټ���ʯī���ϵĵ缫��ӦʽΪ___________________��
�ڽ�ʪ��ĵ��۵⻯����ֽ�������ձ�______���Fe����C�����缫���Ϸ���������ֽ�ȱ�������ɫ��������Ϊ������Cl2���������ɵ�I2������Ӧ��Cl2��I2�����ʵ���֮��Ϊ5��1�������������ᣬ�����Ӧ�Ļ�ѧ����ʽΪ_________________________��
�ۼ������������ȫ���ݳ���Һ�����ҷ�Ӧ��0.01 mol����ת�ƺ�ֹͣʵ�飬��ʱ�ձ�����Һ�����Ϊ100 mL������Һ��Ͼ��Ⱥ��pH = ____________��
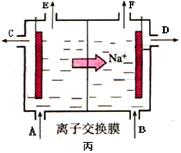
�ܵ����еķ�Ӧ���ڹ�ҵ����ʱ��Ϊ����ֹ��������֮��ķ�Ӧ��ͨ��ʹ�����ͼ��ʾ��װ�ã��������ӽ���Ĥֻ����Na��ͨ����Na�����ƶ�������ͼ�б�ע����H2�ij�����________(�����)��


��1�����ס������ձ���ʢ��CuSO4��Һ��
�ټ��������ϵĵ缫��ӦʽΪ_______________________________________��
����װ�ù���һ��ʱ������ձ��м��������ļ�ʽ̼��ͭ��Cu2(OH)2CO3������ʹ��Һ�ָ�����ʼ״̬����д�����ʱ������װ�÷��������з�Ӧ�Ļ�ѧ����ʽ
________________________________________________________________________��
��2�����ס������ձ���ʢ�ű���NaCl��Һ��
�ټ���ʯī���ϵĵ缫��ӦʽΪ___________________��
�ڽ�ʪ��ĵ��۵⻯����ֽ�������ձ�______���Fe����C�����缫���Ϸ���������ֽ�ȱ�������ɫ��������Ϊ������Cl2���������ɵ�I2������Ӧ��Cl2��I2�����ʵ���֮��Ϊ5��1�������������ᣬ�����Ӧ�Ļ�ѧ����ʽΪ_________________________��
�ۼ������������ȫ���ݳ���Һ�����ҷ�Ӧ��0.01 mol����ת�ƺ�ֹͣʵ�飬��ʱ�ձ�����Һ�����Ϊ100 mL������Һ��Ͼ��Ⱥ��pH = ____________��
�ܵ����еķ�Ӧ���ڹ�ҵ����ʱ��Ϊ����ֹ��������֮��ķ�Ӧ��ͨ��ʹ�����ͼ��ʾ��װ�ã��������ӽ���Ĥֻ����Na��ͨ����Na�����ƶ�������ͼ�б�ע����H2�ij�����________(�����)��
��1����Fe �C 2e��= Fe2����2�֣�
��2CuSO4 + 2H2O 2Cu + O2��+ 2H2SO4��2H2O
2Cu + O2��+ 2H2SO4��2H2O 2H2��+ O2����2�֣�
2H2��+ O2����2�֣�
��2���� O2 + 4e��+ 2H2O = 4OH����2�֣�
�� C��1�֣���5Cl2 + I2 + 6H2O =" 10HCl" + 2HIO3��3�֣�
�� 13��3�֣�
�� F��1�֣�
��2CuSO4 + 2H2O
 2Cu + O2��+ 2H2SO4��2H2O
2Cu + O2��+ 2H2SO4��2H2O 2H2��+ O2����2�֣�
2H2��+ O2����2�֣���2���� O2 + 4e��+ 2H2O = 4OH����2�֣�
�� C��1�֣���5Cl2 + I2 + 6H2O =" 10HCl" + 2HIO3��3�֣�
�� 13��3�֣�
�� F��1�֣�
��

��ϰ��ϵ�д�
�����Ŀ