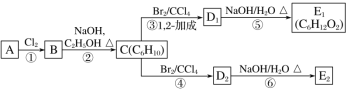
ЬтФПФкШн
ЁОЬтФПЁП(1)дк2LЕФУмБеШнЦїжаЗХШы4mol N2O5ЃЌЗЂЩњШчЯТЗДгІЃК2N2O5(g)![]() 4NO2(g)+O2(g)ЁЃЗДгІжС5minЪБЃЌВтЕУN2O5зЊЛЏСЫ20%ЃЌдђv(NO2)ЮЊ_________ЃЛ5minЪБЃЌN2O5дкЛьКЯЦјЬхжаЕФЬхЛ§ЗжЪ§ЪЧ____ЁЃ
4NO2(g)+O2(g)ЁЃЗДгІжС5minЪБЃЌВтЕУN2O5зЊЛЏСЫ20%ЃЌдђv(NO2)ЮЊ_________ЃЛ5minЪБЃЌN2O5дкЛьКЯЦјЬхжаЕФЬхЛ§ЗжЪ§ЪЧ____ЁЃ
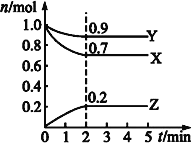
(2)ФГЮТЖШЪБЃЌдквЛИі2LЕФУмБеШнЦїжаЃЌXЁЂYЁЂZШ§жжЮяжЪЕФЮяжЪЕФСПЫцЪБМфБфЛЏЕФЧњЯпШчЭМЫљЪОЁЃИљОнЭМжаЪ§ОнЬюПеЃК
ЂйИУЗДгІЕФЛЏбЇЗНГЬЪНЮЊ______ЁЃ
ЂкШєXЁЂYЁЂZОљЮЊЦјЬхЃЌ2minЪБЗДгІДяЕНЦНКтЃЌДЫЪБЬхЯЕФкбЙЧПгыПЊЪМЪБЕФбЙЧПжЎБШЮЊ____ЁЃ
ЂлШєXЁЂYЁЂZОљЮЊЦјЬхЃЌдђДяЦНКтЪБЃЌШнЦїФкЛьКЯЦјЬхЕФЦНОљЯрЖдЗжзгжЪСПБШЦ№ЪМЭЖСЯЪБ__(ЬюЁАдіДѓЁБЁАМѕаЁЁБЛђЁАЯрЕШЁБ)ЁЃ
ЁОД№АИЁП0.16 molЁЄL-1ЁЄmin-1 61.5% 3X+Y![]() 2Z 9ЁУ10 діДѓ
2Z 9ЁУ10 діДѓ
ЁОНтЮіЁП
БОЬтжївЊПМВьЛЏбЇЦНКтЁЃ
(1) N2O5зЊЛЏСЫ20%ЃЌдђІЄn(N2O5)=4molЁС20%=0.8molЃЌЬхЯЕжаИїзщЗжЕФЮяжЪЕФСПШчЯТБэЫљЪОЃЈЕЅЮЛЃКmolЃЉЃК
2N2O5 | 4NO2 | O2(g) | |
2 | 4 | 1 | |
Ц№ | 4 | 0 | 0 |
зЊ | 0.8 | 1.6 | 0.4 |
ЦН | 3.2 | 1.6 | 0.4 |
ІЄn(NO2)=1.6molЃЌv(NO2)=![]() = 0.16 molЁЄL-1ЁЄmin-1ЃЛИљОнАЂЗќйЄЕТТоЖЈТЩПЩЕУЃКN2O5дкЛьКЯЦјЬхжаЕФЬхЛ§ЗжЪ§ЪЧ
= 0.16 molЁЄL-1ЁЄmin-1ЃЛИљОнАЂЗќйЄЕТТоЖЈТЩПЩЕУЃКN2O5дкЛьКЯЦјЬхжаЕФЬхЛ§ЗжЪ§ЪЧ![]() =61.5%ЃЛ
=61.5%ЃЛ
(2)ЂйЗДгІжаЃЌXЁЂYЕФЮяжЪЕФСПМѕаЁЃЌZЕФЮяжЪЕФСПдіЖрЃЌдђXЁЂYЮЊЗДгІЮяЃЌZЮЊЩњГЩЮяЃЛ0-2minФкЃЌІЄn(X)=0.3molЃЌІЄn(Y)=0.1molЃЌІЄn(Z)=0.2molЃЌЧв2minКѓИїзщЗжЕФЮяжЪЕФСПВЛдйИФБфЃЛЫљвдЃЌИУЗДгІЕФЛЏбЇЗНГЬЪНЮЊ3X+Y![]() 2ZЃЛ
2ZЃЛ
ЂкИљОнРэЯыЦјЬхзДЬЌЗНГЬ:PV=nRTЃЌ2minЪБЬхЯЕбЙЧПгыПЊЪМЪБЕФбЙЧПжЎБШЕШгкСНИіЪБМфЕФЦјЬхЕФзмЮяжЪЕФСПжЎБШЃЌИУБШР§ЮЊЃЈ0.9+0.7+0.2ЃЉЃКЃЈ1.0+1.0ЃЉ=1.8ЃК2=9ЃК10ЃЛ
ЂлИУЗДгІЕФЛЏбЇЗНГЬЪНЮЊ3X+Y![]() 2ZЃЌЗДгІДге§ЗДгІПЊЪМЃЌдђЬхЯЕЕФзмЮяжЪЕФСПМѕаЁЃЌгЩгкЬхЯЕжаИїзщЗжЖМЪЧЦјЬхЃЌдђЬхЯЕЕФзмжЪСПВЛБфЃЌЛьКЯЦјЬхЕФЦНОљЯрЖдЗжзгжЪСПдіДѓЁЃ
2ZЃЌЗДгІДге§ЗДгІПЊЪМЃЌдђЬхЯЕЕФзмЮяжЪЕФСПМѕаЁЃЌгЩгкЬхЯЕжаИїзщЗжЖМЪЧЦјЬхЃЌдђЬхЯЕЕФзмжЪСПВЛБфЃЌЛьКЯЦјЬхЕФЦНОљЯрЖдЗжзгжЪСПдіДѓЁЃ

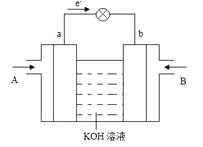
ЁОЬтФПЁПМзДМгУЭОШевцЙуЗКЃЌдНРДдНв§Ц№ЩЬМвЕФЙизЂЃЌЙЄвЕЩЯМзДМЕФКЯГЩЭООЖЖржжЖрбљЁЃдк![]() УмБеШнЦїФкЃЌ
УмБеШнЦїФкЃЌ![]() ЪБЗДгІЃК
ЪБЗДгІЃК![]()
![]() ЃЌЬхЯЕжа
ЃЌЬхЯЕжа![]() ЫцЪБМфЕФБфЛЏШчЯТБэЃК
ЫцЪБМфЕФБфЛЏШчЯТБэЃК
ЪБМф(s) | 0 | 1 | 2 | 3 | 5 |
| 0.020 | 0.011 | 0.008 | 0.007 | 0.007 |
(1)ЭМжаБэЪО![]() ЕФБфЛЏЕФЧњЯпЪЧ________ЁЃ
ЕФБфЛЏЕФЧњЯпЪЧ________ЁЃ
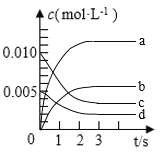
(2)гУ![]() БэЪОДг0~2sФкИУЗДгІЕФЦНОљЫйТЪ
БэЪОДг0~2sФкИУЗДгІЕФЦНОљЫйТЪ![]() ________
________![]() ЁЃ
ЁЃ
(3)ФмЫЕУїИУЗДгІвбДяЕНЦНКтзДЬЌЕФЪЧ________ЁЃ
a.![]() гы
гы![]() ЕФХЈЖШБЃГжВЛБф b.ШнЦїФкУмЖШБЃГжВЛБф
ЕФХЈЖШБЃГжВЛБф b.ШнЦїФкУмЖШБЃГжВЛБф
c.ШнЦїФкбЙЧПБЃГжВЛБф d.УПЯћКФ![]() ЕФЭЌЪБга
ЕФЭЌЪБга![]() аЮГЩ
аЮГЩ
(4)вбжЊГЃЮТГЃбЙЯТ1gвКЬЌМзДМШМЩеЩњГЩ![]() ЦјЬхКЭвКЬЌЫЎЗХГі
ЦјЬхКЭвКЬЌЫЎЗХГі![]() ЕФШШСПЃЌдђИУЗДгІЕФШШЛЏбЇЗНГЬЪНЮЊ________ЁЃ
ЕФШШСПЃЌдђИУЗДгІЕФШШЛЏбЇЗНГЬЪНЮЊ________ЁЃ
(5)![]() гы
гы![]() ЕФЗДгІПЩНЋЛЏбЇФмзЊЛЏЮЊЕчФмЃЌЦфЙЄзїдРэШчЯТЭМЫљЪОЃЌЭМжа
ЕФЗДгІПЩНЋЛЏбЇФмзЊЛЏЮЊЕчФмЃЌЦфЙЄзїдРэШчЯТЭМЫљЪОЃЌЭМжа![]() Дг________(ЬюAЛђB)ЭЈШыЁЃbЕчМЋЗДгІЪНЮЊ________ЁЃ
Дг________(ЬюAЛђB)ЭЈШыЁЃbЕчМЋЗДгІЪНЮЊ________ЁЃ
ЁОЬтФПЁПЯрЭЌЮТЖШЯТЃЌШнЛ§ЯрЭЌЕФМзЁЂввЁЂБћ3ИіКуШнУмБеШнЦїжаОљЗЂЩњЗДгІЃК![]()
![]() ЪЕбщВтЕУгаЙиЪ§ОнШчЯТБэЫљЪОЃК
ЪЕбщВтЕУгаЙиЪ§ОнШчЯТБэЫљЪОЃК
ШнЦїБрКХ | Ц№ЪМИїЮяжЪЕФЮяжЪЕФСП/mol | ДяЕНЦНКтЪБЬхЯЕФмСПЕФБфЛЏ/kJ | ||
|
|
| ||
Мз | 2 | 1 | 0 |
|
вв | 1.8 | 0.9 | 0.2 |
|
Бћ | 0 | 0 | 2 |
|
ЯТСаХаЖЯжае§ШЗЕФЪЧЃЈ ЃЉ
A.![]()
B.ШєЩ§ИпЮТЖШЃЌЗДгІЕФШШаЇгІВЛБф
C.![]()
D.ЩњГЩ![]() ЪБЗХГіЕФШШСПДѓгк98.5 kJ
ЪБЗХГіЕФШШСПДѓгк98.5 kJ
ЁОЬтФПЁП2SO2(g) + O2(g) ![]() 2SO3(g)ЪЧЙЄвЕжЦСђЫсЕФжївЊЗДгІжЎвЛЁЃвЛЖЈЮТЖШЯТЃЌдкМзЁЂввЁЂБћШ§ИіШнЛ§ОљЮЊ2 LЕФКуШнУмБеШнЦїжаЭЖШыSO2(g)КЭO2(g)ЃЌЦфЦ№ЪМЮяжЪЕФСПМАSO2ЕФЦНКтзЊЛЏТЪШчЯТБэЫљЪОЁЃЯТСаХаЖЯжаЃЌе§ШЗЕФЪЧ
2SO3(g)ЪЧЙЄвЕжЦСђЫсЕФжївЊЗДгІжЎвЛЁЃвЛЖЈЮТЖШЯТЃЌдкМзЁЂввЁЂБћШ§ИіШнЛ§ОљЮЊ2 LЕФКуШнУмБеШнЦїжаЭЖШыSO2(g)КЭO2(g)ЃЌЦфЦ№ЪМЮяжЪЕФСПМАSO2ЕФЦНКтзЊЛЏТЪШчЯТБэЫљЪОЁЃЯТСаХаЖЯжаЃЌе§ШЗЕФЪЧ
Мз | вв | Бћ | ||
Ц№ЪМЮяжЪЕФСП | n(SO2) / mol | 0.4 | 0.8 | 0.8 |
n(O2) / mol | 0.24 | 0.24 | 0.48 | |
SO2ЕФЦНКтзЊЛЏТЪ / % | 80 | ІС1 | ІС2 | |
A. МзжаЗДгІЕФЦНКтГЃЪ§аЁгквв
B. ЦНКтЪБЃЌБћжаc(SO3)ЪЧМзжаЕФ2БЖ
C. ИУЮТЖШЯТЃЌЦНКтГЃЪ§жЕЮЊ400
D. ЦНКтЪБЃЌМзжаO2ЕФзЊЛЏТЪДѓгкввжаO2ЕФзЊЛЏТЪ