��Ŀ����
16����1������4�����ʾ���һ�����ʵ����������3�ֲ�ͬ��Ϊ��A��CaO��Na2O��CO2��CuO
B��������������Һ��ʯ���顢��
C��Na��Fe��Cu��Zn
D��HCl��HClO4��H2SO4��HNO3
ACO2��Bʯ���飻CCu��DHCl��
��2����ˮ�� ���ռ� �۴���ʯ ���Ȼ��ƾ��� ������ �ް��� ������ ������ ��Һ̬�Ȼ��� ��������Һ
�����������ܵ�����Ǣ٢ݢ⣬���ڵ���ʵ��Ǣڢۢܢᣬ���ڷǵ���ʵ��Ǣޣ�
��3����Ҫ��д�����з���ʽ��
��Fe2��SO4��3��ˮ�еĵ��뷽��ʽ��Fe2��SO4��3=2Fe3++3SO42-��
��ʵ������CO2��������ӷ���ʽ��CaCO3+2H+=Ca2++2H2O��
����NaHSO4��Һ�м����п�����ӷ���ʽ��Zn+2H+=H2��+Zn2+��
��4��CO32-+2H+�TCO2��+H2O��д����Ӧ�Ļ�ѧ����ʽ��Na2CO3+2HCl�TCO2��+H2O+2NaCl��
���� ��1��A��CO2Ϊ�ǽ������������Ϊ���������
B��ʯ����Ϊ��Һ������Ϊ���壻
C��Cu�����û����е������ӣ�
D��HCl�����
��2�����ʵ�����������������ɵ��Ӻ������ƶ������ӣ�
���ݵ������������״̬��ˮ��Һ���ܵ���Ļ���������ᡢ��Ρ�����������Ȼ����
�ǵ����������״̬��ˮ��Һ�ж����ܵ���Ļ���������ǽ��������ˮ���⣩�������������л��������ǡ��Ҵ��ȣ�
��3����Fe2��SO4��3��ǿ����ʣ���ȫ�����������������Ӻ���������ӣ�
��̼��������ᷴӦ�����Ȼ��ƺͶ�����̼��ˮ��
����NaHSO4��Һ�м����п�ۣ���������п��Ӧ����п���Ӻ�������
��4��CO32-+2H+�TCO2��+H2O��ʾ������̼������ǿ�����ǿ�����ʽ�η�Ӧ���ɶ�����̼��ˮ���������Σ�
��� �⣺��1��A��CaO��Na2O��CuO���ǽ����������CO2Ϊ�ǽ���������ʴ�Ϊ��CO2��
B��ʯ����Ϊ��Һ��������������Һ������Ϊ���壬�ʴ�Ϊ��ʯ���飻
C��Cu�����û����е������ӣ�Na��Fe��Zn�Ļ����Խ�ǿ�����û������е������ӣ��ʴ�Ϊ��Cu��
D��HCl������Ԫ��Ϊ�����ᣬHClO4��H2SO4��HNO3Ϊ�����ᣬ�ʴ�Ϊ��HCl��
��2����ˮ���ǵ��ʣ��Ȳ��ǵ����Ҳ���Ƿǵ���ʣ��������ɵ��ӣ��ܹ����磻
���ռ�������״̬��ˮ��Һ���ܵ���Ļ�������ڵ���ʣ����������ƶ������Ӻ͵��ӣ������磻
�۴���ʯ������״̬���ܹ����磬���ڵ���ʣ����Dz��������ƶ������Ӻ͵��ӣ������磻
���Ȼ��ƾ��壬������״̬��ˮ��Һ���ܵ���Ļ�������ڵ���ʣ����������ƶ������Ӻ͵��ӣ������磻
���������ڻ����Ȳ��ǵ����Ҳ���Ƿǵ���ʣ����������ƶ������ӣ��ܹ����磻
�ް������������ܵ�����������ƶ������ӣ������磬���ڷǵ���ʣ�
������������״̬��ˮ��Һ�ж����ܵ���Ļ�����������ɵ��ӻ��������ƶ������ӣ������磻
�������ǵ��ʣ��Ȳ��ǵ����Ҳ���Ƿǵ���ʣ����������ɵ��ӻ��������ƶ������ӣ����ܹ����磻
��Һ̬�Ȼ�����ˮ��Һ���ܵ���Ļ�������ڵ���ʣ����������ɵ��ӻ��������ƶ������ӣ����ܹ����磻
��������Һ�����ڻ����Ȳ��ǵ����Ҳ���Ƿǵ���ʣ����������ƶ������ӣ��ܹ����磻
�����ܵ�����ǣ��٢ݢ⣻���ڵ���ʵ��ǣ��ڢۢܢ���ڷǵ���ʵ��ǣ��ޣ�
��3����Fe2��SO4��3��ˮ�еĵ��뷽��ʽ��Fe2��SO4��3=2Fe3++3SO42-��
�ʴ�Ϊ��Fe2��SO4��3=2Fe3++3SO42-��
��ʵ������CO2��������ӷ���ʽ��CaCO3+2H+=Ca2++2H2O��
�ʴ�Ϊ��CaCO3+2H+=Ca2++2H2O��
����NaHSO4��Һ�м����п�����ӷ���ʽ��Zn+2H+=H2��+Zn2+��
�ʴ�Ϊ��Zn+2H+=H2��+Zn2+��
��4��CO32-+2H+�TCO2��+H2O���Ա�ʾ̼���������ᷴӦ����ѧ����ʽ��Na2CO3+2HCl�TCO2��+H2O+2NaCl��
�ʴ�Ϊ��Na2CO3+2HCl�TCO2��+H2O+2NaCl��
���� ���⿼�������ʵķ��ࡢ����ʡ��ǵ���ʵ��жϣ����ӷ���ʽ����д����ȷ��ظ�����ӷ���ʽ��д�ķ����ǽ���ؼ�����Ŀ�ѶȲ���

| A�� | �����Ũ�ȴﵽ1 mol•L-1 | |
| B�� | H+��Ũ�ȴﵽ0.5 mol•L-1 | |
| C�� | ������ӵ�Ũ�ȡ���������ӵ�Ũ�ȡ�H+��Ũ�Ⱦ�Ϊ0.5 mol•L-1 | |
| D�� | ������ӵ�������ӵ����ʺ��������½�ϳɴ�����ӵ�������ȣ� |
| A�� | Na��Mg��Al��Si | B�� | Na��Be��B��C | C�� | P��S��Cl��Ar | D�� | C��N��O��F |
| A�� | C2H5OH��l��+3O2��g���T2CO2��g��+3H2O��g����H=-1367.0 kJ/mol��ȼ���ȣ� | |
| B�� | S��s��+O2��g���TSO2��g����H=-269.8kJ/mol����Ӧ�ȣ� | |
| C�� | NaOH��aq��+HCl��aq���TNaCl��aq��+H2O��l����H=+57.3kJ/mol���к��ȣ� | |
| D�� | 2NO2�TO2+2NO��H=+116.2kJ/mol����Ӧ�ȣ� |
| A�� | ������Һ��һ����c��H+��=c��OH-��=1��10-7mol/L | |
| B�� | ��0.1mol/L��NaOH��Һ�м�����������ˮ����c��H+����c��OH-�������� | |
| C�� | 25��ʱ��pH=6����Һ�в����ܴ���NH3•H2O���� | |
| D�� | �����£�pH=8����Һ�п��ܴ���CH3COOH���� |
| A�� | v��D��=0.6 mol•L-1•min-1 | B�� | v��B��=0.45 mol•L-1•s-1 | ||
| C�� | v��C��=0.40 mol•L-1•min-1 | D�� | v��A��=0.2 mol•L-1•s-1 |
| A�� | ��ȥ���ӳɡ�ˮ�� | B�� | �ӳɡ���ȥ��ȡ�� | C�� | ��ȥ���ӳɡ���ȥ | D�� | ȡ������ȥ���ӳ� |
 ����������Ӧ�IJ��
����������Ӧ�IJ��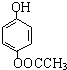 ��
��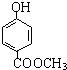 ��
��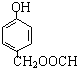 ������֮һ����
������֮һ����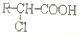

 ��
��