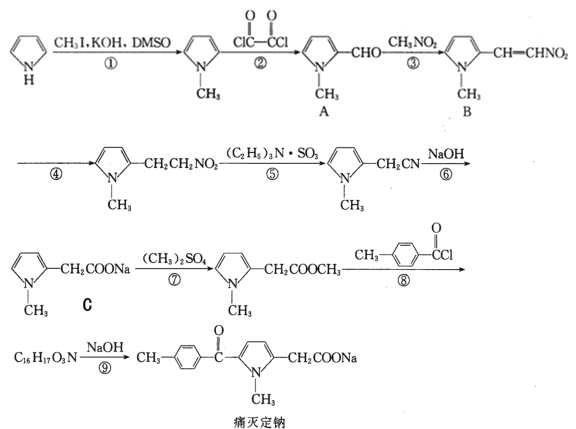
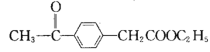
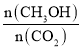��Ŀ����
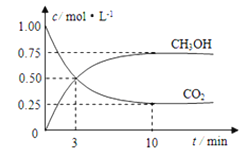
����Ŀ��һ���¶��£���1.0L���ܱ������м���0.60molX��g����������ӦX(g)![]() Y(s)+2Z(g)����÷�Ӧ��X��Ũ���뷴Ӧʱ��Ĺ�ϵ�����ʾ��
Y(s)+2Z(g)����÷�Ӧ��X��Ũ���뷴Ӧʱ��Ĺ�ϵ�����ʾ��
��Ӧʱ��t/min | 0 | 1 | 2 | 3 | 4 | 6 | 8 |
c(X)/(mol��L-1) | 0.60 | 0.42 | 0.30 | 0.21 | 0.15 | a | 0.0375 |
(1)0��3min����Z��ʾ��ƽ����Ӧ�ٶ�v(Z)=___��
(2)�����÷�Ӧ�з�Ӧ���Ũ����ʱ��Ĺ�ϵ���ó��Ľ�����___���ɴ˹����Ƴ���6minʱ��Ӧ��X��Ũ��Ϊ___mol��L-1��
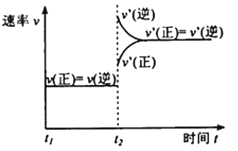
(3)�÷�Ӧ���淴Ӧ������ʱ��仯��������ͼ��ʾ��t2ʱ�ı������������___��___��

���𰸡�0.26mol��L-1��min-1 ÿ��2minX��Ũ�ȼ���Ϊԭ����һ�� 0.075 ����Z ������ϵ��ѹǿ
��������
(1)0��3min�ڿ������X��ʾ��ƽ����Ӧ���ʣ�Ȼ�����û�ѧ��������ϵ�����Z��ʾ��ƽ����Ӧ�ٶ�v(Z)��
(2)�����÷�Ӧ�з�Ӧ���Ũ����ʱ��Ĺ�ϵ��Ѱ�ҹ������ݵĹ����ԣ��ɴ˵ó��Ľ��ۡ��ɴ˹��ɿ��Ƴ���6minʱ��Ӧ��X��Ũ�ȡ�
(3)���ݷ�Ӧ��t2ʱ�ı��������Ũ�ȡ�ѹǿ���¶ȡ��������������з�����
(1)0~3min�ڣ�c(X)=(0.60-0.21)mol/L=0.39mol/L��ƽ����Ӧ����v(X)=![]() = 0.13mol��L-1��min-1����v(Z)=2v(X)����v(Z)=0.26mol��L-1��min-1����Ϊ��0.26mol��L-1��min-1��
= 0.13mol��L-1��min-1����v(Z)=2v(X)����v(Z)=0.26mol��L-1��min-1������0.26mol��L-1��min-1��
(2)���ݱ������ݿ�֪��ÿ��2min��X��Ũ�ȼ���Ϊԭ����һ�룬�ɴ˹����Ƴ���6minʱ��Ӧ��X��Ũ��Ϊ0.075 mol��L-1����Ϊ��0.075��
(3)![]() ʱ�̣�
ʱ�̣�![]() ˲�������ܵ�ԭ���Ǽ���������Z��������ϵ��ѹǿ����Ϊ������Z��������ϵ��ѹǿ��
˲�������ܵ�ԭ���Ǽ���������Z��������ϵ��ѹǿ����Ϊ������Z��������ϵ��ѹǿ��