��Ŀ����
����Ŀ��Ϊ��Ч�������������ػ�����ȡ��ʩ���ƴ����������о�����Ч���ƿ����еĵ������̼������ĺ����Ե���Ϊ��Ҫ��
I.���������о�
��1��һ�������£���2molNO��2molO2���ں����ܱ������з�����Ӧ2NO(g)+O2(g)![]() 2NO2(g)�����и�����˵����Ӧ�ﵽƽ��״̬���� ________________������ĸ���ţ� ��
2NO2(g)�����и�����˵����Ӧ�ﵽƽ��״̬���� ________________������ĸ���ţ� ��
a����ϵѹǿ���ֲ��� b�����������ɫ���ֲ���
c��NO��O2�����ʵ���֮�ȱ��ֲ��� d��ÿ����1 molO2ͬʱ����2 molNO2
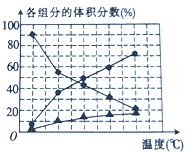
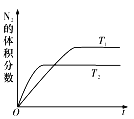
��2��������ȼ������ʱ������N2��O2�ķ�Ӧ��N2 +O2![]() 2NO���ǵ�������β���к���NO��ԭ��֮һ����T1��T2�¶��£�һ������NO�����ֽⷴӦʱN2�����������ʱ��仯����ͼ��ʾ������ͼ���жϷ�ӦN2(g)+O2(g)
2NO���ǵ�������β���к���NO��ԭ��֮һ����T1��T2�¶��£�һ������NO�����ֽⷴӦʱN2�����������ʱ��仯����ͼ��ʾ������ͼ���жϷ�ӦN2(g)+O2(g)![]() 2NO(g)����H__________0(����������������)��
2NO(g)����H__________0(����������������)��
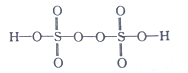
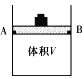
����̼�������о�
��1������ɱ䣨����������֮���Ħ�������Բ��ƣ����ܱ���������ͼ��ʾ���ֽ�3molH2��2molCO���������У��ƶ����������VΪ2L����í���̶���A��B�㣬�����ϳɼ״��ķ�Ӧ���£�CO(g)+2H2(g)![]() CH3OH(g)��
CH3OH(g)��
�ⶨ��ͬ��������ͬʱ����ڵ�CO��ת���ʣ��õ��������ݣ�
T������ | 10min | 20min | 30min | 40min |
T1 | 20% | 55% | 65% | 65% |
T2 | 35% | 50% | a1 | a2 |
�������ϱ����ݣ���Ƚ�T1_________T2(ѡ����>������<������=��)��T2���£���30min ʱ��a1=________�����¶��µĻ�ѧƽ�ⳣ��Ϊ__________________��
��T2���£���40minʱ����ȥí���������ܷ������ã�����û�з����ƶ�������������ͨ��6molCO����ʱv(����________v(�棩(ѡ����>������<������=��)��
��2��һ�������¿��ü״���CO��Ӧ���ɴ�������CO��Ⱦ�������£���a mol/L�Ĵ�����b mol/L Ba(OH)2��Һ��������(��Ϻ���Һ����仯���Բ��ƣ�����ַ�Ӧ����Һ�д���2c(Ba2+)��c(CH3COO-)���������Һ�еĵ��ƽ���֪����Һ��pH=___________��������������ĵ��볣��Ka =____________ (�ú�a��b�Ĵ���ʽ��ʾ����
���𰸡� abc > < 50% 4��4(mol/L)-2 ��4(L/mol)2 < 7 2��10-7b/(a-2b) mol/L
����������(1)�����ܱ������з�����Ӧ��2NO(g)+O2(g)2NO2(g)��a����Ӧ�Ǹ���������ı�ķ�Ӧ������������ѹǿ�ı䣬ƽ��ʱ����ϵѹǿ���ֲ��䣬����ȷ��b�����������ɫ���ֲ��䣬˵������������Ũ�ȱ��ֲ��䣬�ﵽƽ�⣬����ȷ��c��NO��O2����ʼ���ʵ�����ȣ�����ѧ��������ͬ���仯���Ͳ���ͬ�����û�дﵽƽ�⣬NO��O2 �����ʵ���֮�Ȼᷢ���ı䣬�������ı�˵��������ƽ�⣬����ȷ��d��O2 �Ƿ�Ӧ�NO2��������������κ�ʱ����ÿ����1 molO2ͬʱ����2 molNO2���ʴ���ѡ��abc��
(2)��ͼ��֪�ȴﵽƽ������ʱ��϶̵��¶Ƚϸߣ��Ҵ��¶��µ��������������С��˵���¶����ߺ�Ӧ�����ƶ����ʷ�ӦΪ���ȷ�Ӧ���ʴ�Ϊ������
����(1)�������¶ȣ���ѧ��Ӧ���ʼӿ죬10min�ڣ�T2ʱCOת���ʴ���T1ʱ����T1��T2��
T2���£�10minʱCO2ת����Ϊ35%��20minʱCO2ת����Ϊ50%��10-20minֻת��15%��˵��20minʱ�Ѵ�ƽ��״̬���ʵ�30minʱ��CO2ת����Ϊ50%��
T2���£�CO(g)+2H2(g) ![]() CH3OH(g)��
CH3OH(g)��
��ʼ(mol) 2 3 0
ת��(mol) 1 2 1
\ƽ��(mol) 11 1
��ѧƽ�ⳣ��K=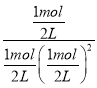 =4���ʴ�Ϊ������50%��4��
=4���ʴ�Ϊ������50%��4��
�ڰ�ȥí������������ͬ��ͬѹ�½��У����֮�ȵ������ʵ���֮�ȣ�������6molCO������ƽ�ⲻ�ƶ�����ʱ���������Ϊ6L��Qc=![]() =
=![]() ��K����ƽ�������ƶ���v(��)��v(��)���ʴ�Ϊ������
��K����ƽ�������ƶ���v(��)��v(��)���ʴ�Ϊ������
(2)��Ӧƽ��ʱ��2c(Ba2+)=c(CH3COO-)=bmol/L���ݵ���غ㣬��Һ��c(H+)=c(OH-)=10-7mol/L����Һ�����ԣ��������ƽ�ⳣ�����ݵ��뷽��ʽд��K=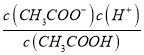 =
=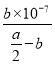 =
=![]() ��107mol/L���ʴ�Ϊ��7��
��107mol/L���ʴ�Ϊ��7�� ![]() ��107mol/L��
��107mol/L��

����Ŀ����10 L�����ܱ������г���X(g)��Y(g)��������ӦX(g) + Y(g)![]() M(g) +N(g)������ʵ���������±���ʾ��
M(g) +N(g)������ʵ���������±���ʾ��
ʵ���� | �¶�/�� | ��ʼʱ���ʵ���/mol | ƽ��ʱ���ʵ���/mol | |
n(X) | n(Y) | n(M) | ||
�� | 700 | 0.40 | 0.10 | 0.090 |
�� | 800 | 0.10 | 0.40 | 0.080 |
�� | 800 | 0.20 | 0.30 | a |
�� | 900 | 0.10 | 0.15 | b |
����˵����ȷ����
A. ʵ�����У���5 minʱ���n(M)=0.050 mol����05 min�ڣ���N��ʾ��ƽ����Ӧ����v(N) =1.0��10-2 mol/(L min)
B. ʵ�����У��÷�Ӧ��ƽ�ⳣ��K=2.0
C. ʵ�����У���Ӧ�ﵽƽ��ʱ��X��ת����Ϊ40%
D. ʵ�����У���Ӧ�ﵽƽ��ʱ��b<0.060