��Ŀ����
5������18.4mol/L��ŨH2SO4������500mL0.2mol/L��ϡH2SO4���ɹ�ѡ��������У��ٲ����� ����ƿ ���ձ� �ܽ�ͷ�ι� ����Ͳ ��������ƽ ��ҩ�ף���ش��������⣺
��1�����������У�������ϡH2SOʱ����Ҫʹ�õ��Тޢߡ�������ţ�����ȱ�ٵ�������500mL����ƿ��
��2����ʵ���ʵ�鲽�裺�ټ��㣬��Ũ����V=5.4mL������ȡ����ϡ����ȴ������Һ����ϴ�ӣ����ݣ���ҡ�ȣ���װƿ������ǩ��
��ȡŨ����ʱӦѡ�â٣�ѡ���10mL����50mL����100mL���ֹ������ţ�����Ͳ��
��3�����в���ʹ������ҺŨ��ƫ�͵���BC
A������ƿ�е�ԭ������ˮδ��ȥ
B��ϡ���õ��ձ�δ��ϴ��
C������ʱ�۲�Һ������
D���ý�ͷ�ι�������ƿ��ˮʱ��ˮδ���̶���ֹͣ��ˮ��
���� ��1����������һ�����ʵ���Ũ����Һ�IJ�������ѡȡʵ��������
��2��������Һϡ�������������ʵ����ʵ������������ҪŨ�������������Ũ�������ѡ����ʵ���Ͳ��
��������һ�����ʵ���Ũ����Һ�IJ���������
��3����������һ�����ʵ���Ũ����Һ�IJ��������жϣ������������������ʵ����ʵ�������Һ�������Ӱ�죬����C=$\frac{n}{V}$������������
��� �⣺��1������һ�����ʵ���Ũ��������Һ��һ�㲽���У���ȡ��ϡ�͡���Һ��ϴ�ӡ����ݡ�ҡ�ȵȲ�����һ������Ͳ��ȡŨ������Һ���ձ���ϡ�ͣ���ȴ��ת�Ƶ�500mL����ƿ�У����ò���������������ˮ��Һ�����̶���1��2cmʱ�����ý�ͷ�ιܵμӣ�������Ҫ�������в���������Ͳ����ͷ�ιܡ�С�ձ���500ml����ƿ�������ò�����������������ƽ��ҩ�ף���ȱ�ٵ�����Ϊ��500ml����ƿ��
�ʴ�Ϊ���ޢߣ�500ml����ƿ��
��2������ҪŨ�������ΪV����������Һϡ�������������ʵ����ʵ������䣺V��18.4mol/L=0.5L��0.2mol/L�����V=0.0054L����5.4mL������һ�����ʵ���Ũ��������Һ��һ�㲽���У���ȡ��ϡ�͡���Һ��ϴ�ӡ����ݡ�ҡ�ȵȣ���ȡ5.4mL��Һ��Ӧѡ��10mL��Ͳ��
�ʴ�Ϊ��5.4����ȡ����Һ��ϴ�ӣ����ݣ��٣�
��3��A������ƿ�е�ԭ������ˮδ��ȥ�������ʵ����ʵ�������Һ��������������Ӱ�죬��ҺŨ�Ȳ��䣬��A��ѡ��
B��ϡ���õ��ձ�δ��ϴ�ӣ��������ʵ����ʵ���ƫС����ҺŨ��ƫС����Bѡ��
C������ʱ�۲�Һ�����ӣ�������Һ���ƫ����ҺŨ��ƫ��Cѡ��
D���ý�ͷ�ι�������ƿ��ˮʱ��ˮδ���̶���ֹͣ��ˮ��������Һ���ƫС����ҺŨ��ƫ��D��ѡ��
��ѡ��BC��
���� ���⿼����һ�����ʵ���Ũ����Һ�����ƣ���Ϥ����ԭ�������ǽ���ؼ���ת������ƿ��ѡ���ʹ��ע�����ע�����ķ�����

 Сѧ�̲���ȫ���ϵ�д�
Сѧ�̲���ȫ���ϵ�д�| A�� | CH3OH | B�� | C2H5OH | C�� | C3H7OH | D�� | C4H9OH |
| A�� | ̼��������Һ�м�����NaOH��Һ��HCO3-+OH-�TCO2 ��+H2O | |
| B�� | �Ȼ�����Һ�м���������������ƣ�Al3++OH-�TAl��OH��3 �� | |
| C�� | ����������ˮ��Ӧ��2Na2O2 +2H2O�T4Na++4OH-+O2 �� | |
| D�� | �����������ᷴӦ��Al2O3+6H+�T2Al3++3H2O |
| A�� | ��Ȼ����Ϊ����ԭ����Ҫ���ںϳɰ��������״� | |
| B�� | ú����ֱ��Һ����ú��������������Һ��ȼ�� | |
| C�� | ú���Ե���̼Ϊ���ĸ��ӻ�������ʱ����̼���ϵ����ʷ�����ѧ�仯 | |
| D�� | ����ϩ���ϵ���Ҫ�ɷ־���ϩ������ϩͨ���ۺϷ�Ӧ�Ƶ� |
| A�� | CH4��Ħ������Ϊ16g | |
| B�� | 3.01��1023��SO2���ӵ�����Ϊ32g | |
| C�� | ��״���£�1mol�κ����������Ϊ22.4L | |
| D�� | Ħ���ǹ��ʵ�λ�����߸�����������֮һ |
 �״�-����ȼ�ϵ�أ�DMFC����һ�ָ�Ч�ܡ�����Ⱦ�ij��ص�أ��乤��ԭ����ͼ�������й�������ȷ���ǣ�������
�״�-����ȼ�ϵ�أ�DMFC����һ�ָ�Ч�ܡ�����Ⱦ�ij��ص�أ��乤��ԭ����ͼ�������й�������ȷ���ǣ�������| A�� | �����ĵ缫��ӦʽΪ��CH3OH+H2O-6e-�TCO2+6H+ | |
| B�� | H+��������ͨ������Ĥ������ | |
| C�� | d��������CO2 | |
| D�� | ͼ��b��c�ֱ���O2���״� |
| A�� | ͨ�����O2 | B�� | ��С��ϵѹǿ | C�� | ��ȥ����SO3 | D�� | ������ϵ�¶� |


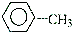
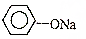
 CH3OH��һ����ɫ�д̼�����ζ��Һ�壬����������������Ҫ��;��ͬʱҲ��һ����Ҫ�Ļ���ԭ�ϣ�
CH3OH��һ����ɫ�д̼�����ζ��Һ�壬����������������Ҫ��;��ͬʱҲ��һ����Ҫ�Ļ���ԭ�ϣ�