��Ŀ����
1��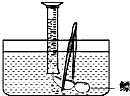 С��������ɱ��ʱ�������ȡ��������������Ȥ����ȷ���ˡ�̽��������������ͳɷ֡���Ϊ�о���ѧϰ�Ŀ��⣮С��ͨ�������й����ϻ�֪����������������Լռ$\frac{1}{4}$��������Ҫ�Ƕ�����̼��������̼������ˮ���͵�����̽�����������У�
С��������ɱ��ʱ�������ȡ��������������Ȥ����ȷ���ˡ�̽��������������ͳɷ֡���Ϊ�о���ѧϰ�Ŀ��⣮С��ͨ�������й����ϻ�֪����������������Լռ$\frac{1}{4}$��������Ҫ�Ƕ�����̼��������̼������ˮ���͵�����̽�����������У���1�������������������С����������ַ�����
A����ҽ��ע������ȡ�������壬�����������
B����ˮ��������������ˮ�������ռ��������岢�������������ͼ����
����Ϊ���ַ����к�����A��
��2��̽����������ijɷ֣�����������ƿ���������壬������������ʵ����֤���裮
| ��֤ | �� �� | �� �� |
| ������ | ��ȼ�ŵ�ľ�����뺬������������ļ���ƿ�� | ľ���ܼ���ȼ�� |
| ��������̼ | �ں�������������ļ���ƿ�м��������ij���ʯ��ˮ | ʯ��ˮ����� |
���� ��1��̽��������������ͳɷ֣��������̼������ˮ������ѡ��ˮ���ռ���
��2��������������������ȼ�ԣ�
���������̼����������ʹ����ʯ��ˮ����ǵ����ʣ�
��� �⣺��1��������̼����ˮ�����ڵĶ�����̼����������ˮ���ռ������������ʵ�����С����������ˮ���ռ�������ҽ��ע������ȡ�������壬��������������������ˮ���ռ���ȱ�㣬
�ʴ�Ϊ��A��
��2������������ȼ�����ԣ�ȼ�ŵ�ľ������ƿ��ľ�����Լ���ȼ�գ�������̼����ʹ����ʯ��ˮ����ǣ�������ʯ��ˮ��������̼��
�ʴ�Ϊ��
| ʵ��Ŀ�� | ���� | ���� |
| ��ȼ�ŵ�ľ�����뺬������������ļ���ƿ�� | ľ���ܼ���ȼ�� | |
| �ں�������������ļ���ƿ�м��������ij���ʯ��ˮ | ʯ��ˮ����� |
���� ���⿼��ʵ��װ�ü����ʵļ���ͼ��飬Ϊ��Ƶ���㣬������������ʡ����鷽��Ϊ���Ĺؼ������ط�����Ӧ�������Ŀ��飬��Ŀ�ѶȲ���

��ϰ��ϵ�д�
�����Ŀ
11��������ʵ��̽��ͬһ����Ԫ�����ʵĵݱ����
��1��̽���������﮵�綠����Եĵݱ���ɣ�ͨ�����Na��K������ˮ��Ӧ�Ա���˵����������K��ˮ��Ӧ�������Ѹ���ۻ���һС���Ĵ��ζ�����������ը��������ɫ�����������Ӧ�Ļ�ѧ����ʽΪ2K+2H2O=2KOH+H2������Na��ˮ��Ӧ�����ң�
��2��̽��±�ش�F��I�ķǽ����Եĵݱ����
ijѧ����������µ�ʵ�鷽�����������ѧ�����������ʵ�鱨�森
��ʵ����Ʒ
�������Թܡ���ͷ�ι�
ҩƷ�����Ƶ���ˮ���Ȼ�����Һ���廯����Һ��KI��Һ�����Ȼ�̼
��ʵ�����ݣ����±����յĸ�����д������ݣ�
��1��̽���������﮵�綠����Եĵݱ���ɣ�ͨ�����Na��K������ˮ��Ӧ�Ա���˵����������K��ˮ��Ӧ�������Ѹ���ۻ���һС���Ĵ��ζ�����������ը��������ɫ�����������Ӧ�Ļ�ѧ����ʽΪ2K+2H2O=2KOH+H2������Na��ˮ��Ӧ�����ң�
��2��̽��±�ش�F��I�ķǽ����Եĵݱ����
ijѧ����������µ�ʵ�鷽�����������ѧ�����������ʵ�鱨�森
��ʵ����Ʒ
�������Թܡ���ͷ�ι�
ҩƷ�����Ƶ���ˮ���Ȼ�����Һ���廯����Һ��KI��Һ�����Ȼ�̼
��ʵ�����ݣ����±����յĸ�����д������ݣ�
| ���� | ʵ�鷽�� | ʵ�������������ģ� | ���ӷ���ʽ |
| �� | �����Թ��м����廯����Һ2ml���ټ���4-5��������ˮ��������ݣ�ȡһ���ּ���CCl4�������� | �ϲ�Ϊdz��ɫ������ɫ�����²�Ϊ��ɫ | Cl2+2Br-=Br2+2Cl- |
| �� | ȡ����KI��Һ���Թܣ����뼸�β�������µ���һ����Һ�����ּ���CCl4�������� | �ϲ�Ϊdz��ɫ������ɫ�����²�Ϊ�Ϻ�ɫ | Br2+2I-=I2+2Br- |
| ���� | �����ԣ�Cl2��Br2��I2���ǽ����ԣ�Cl��Br��I�� | ||
9�������ʽ��з�����ѧϰ��ѧ��һ�ַ�����������衱��ѩ�����ϡ���������ˮ���͡���Ȫˮ�������ڣ�������
| A�� | ������ | B�� | ����� | C�� | ������ | D�� | ���ж� |
6������˵����ȷ���ǣ�������
| A�� | C8H10����������ͬ���칹����3�� | |
| B�� | �ṹΪ��-CH=CH-CH=CH-CH=CH-CH=CH-���ĸ߷��ӻ�����䵥������ϩ | |
| C�� | ������һ��ʱ����Ȳ����ȩ���۰�ʲô������ϣ���ȫȼ������������������CO2������ | |
| D�� | ��ϩ�ᣨCH2=CHCOOH����ɽ���ᣨCH3CH=CHCH=CHCOOH������ͬϵ�������������ַ�Ӧ��IJ�����ͬϵ�� |
13���������ӷ���ʽ���ܽ��Ͷ�Ӧʵ����ʵ���ǣ�������
| A�� | ��С�մ���Һ�μ��������HCO3-+H+�T+H2O+CO2�� | |
| B�� | ��Ư��Һ���չ���SO2��SO2+H2O+ClO-=2H++SO42-+Cl- | |
| C�� | ��������ϩ����ͨ������KMnO4��Һ��CH2=CH2+4H++MnO4-�TC2O42-+Mn2++4H2O | |
| D�� | ��ͭ˿����ϡ�����У�3Cu+8H++2NO3-�T3Cu2++2NO��+4H2O |
10������˵��������ǣ�������
| A�� | ˫ԭ�ӵ��ʷ����еĹ��ۼ�һ���ǷǼ��Լ� | |
| B�� | �ڹ��ۻ�������һ�����й��ۼ� | |
| C�� | �������Ӽ��Ļ�����һ�������ӻ����� | |
| D�� | ���й��ۼ��Ļ�����һ���ǹ��ۻ����� |
 ��B�Ľṹ��ʽΪ��
��B�Ľṹ��ʽΪ�� ��
��