��Ŀ����
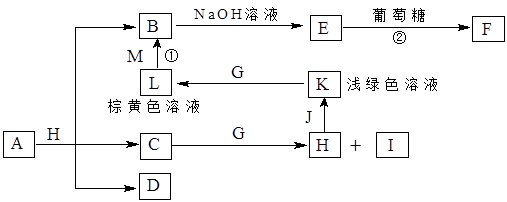
��A��B��C��D���ֶ����ڵķǽ���Ԫ��(�䵥��Ҳ�ɷֱ���A��B��C��D��ʾ)������Ԫ�ص�ԭ��������B��D��C��A˳������D��CԪ�������ڱ���λ�����ڡ���һ�������£�B���Էֱ��A��C��D�������ɼס��ҡ��������C��D���Ͽɵö�����֪�ҡ������������и�����10�����ӣ����Ҽס��ҡ����������������µı仯��ϵ��

����д���пո�
(1)��Ũ��Һ��һ�ֺ�ɫ��ĩ���ȿɵ�A���÷�Ӧ�����ӷ���ʽΪ��
__________________________________________________________��
(2)д�����л�ѧ����õ���ʽ��ʾ�ҷ��ӵ��γɹ���_______________��
����ӵĽṹʽ��__________���ͱ���Ӧ����ĵ���ʽ __________��
(3)д�����з�Ӧ�Ļ�ѧ����ʽ��
��+����D + �ң�_____________;A+����D + �ף�__________________

����д���пո�
(1)��Ũ��Һ��һ�ֺ�ɫ��ĩ���ȿɵ�A���÷�Ӧ�����ӷ���ʽΪ��
__________________________________________________________��
(2)д�����л�ѧ����õ���ʽ��ʾ�ҷ��ӵ��γɹ���_______________��
����ӵĽṹʽ��__________���ͱ���Ӧ����ĵ���ʽ __________��
(3)д�����з�Ӧ�Ļ�ѧ����ʽ��
��+����D + �ң�_____________;A+����D + �ף�__________________
��1��MnO2 + 4HCl(Ũ) 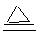 MnCl2 + Cl2�� + 2H2O ��2�֣�
MnCl2 + Cl2�� + 2H2O ��2�֣�
��2��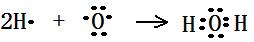 ��2�֣�, H-O-Cl ��2�֣�,NH4Cl�ĵ���ʽ�� ��2�֣�
��2�֣�, H-O-Cl ��2�֣�,NH4Cl�ĵ���ʽ�� ��2�֣�
(3)4NH3 + 6NO = 5N2 + 6H2O ��2�֣� 3Cl2 + 2NH3 = N2 + 6HCl ��2�֣�
 MnCl2 + Cl2�� + 2H2O ��2�֣�
MnCl2 + Cl2�� + 2H2O ��2�֣���2��
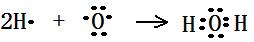 ��2�֣�, H-O-Cl ��2�֣�,NH4Cl�ĵ���ʽ�� ��2�֣�
��2�֣�, H-O-Cl ��2�֣�,NH4Cl�ĵ���ʽ�� ��2�֣� (3)4NH3 + 6NO = 5N2 + 6H2O ��2�֣� 3Cl2 + 2NH3 = N2 + 6HCl ��2�֣�
��

��ϰ��ϵ�д�
�����Ŀ
 ��X����X������ͼ��ʾ��ʵ�飺
��X����X������ͼ��ʾ��ʵ�飺
 ��A��B��Ӧ�Ļ�ѧ����ʽ ��
��A��B��Ӧ�Ļ�ѧ����ʽ �� ��
��