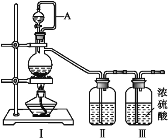
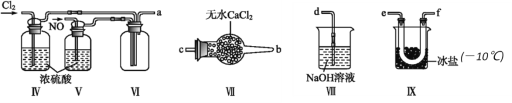
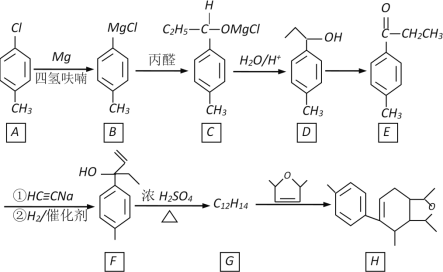
��Ŀ����
����Ŀ�������£�����Ũ�ȵ����������HA��HB��NaHCO3��Ӧ�ų�CO2�������ʱ��Ĺ�ϵ��ͼ��ʾ��������˵��������ǣ�( )

A. ���ԣ�HA<HB
B. pH��Ϊ4��HA��HB��Һ�к͵���NaOHʱ������HA��Һ���С
C. pH��Ϊ9��NaA��Һ��NaB��Һ��ȣ�NaA��Һ��ˮ�ĵ���̶ȴ�
D. Ũ�Ⱦ�Ϊ0.1mol/L��NaA��Һ��NaB��Һ��ȣ�NaA��Һ��ˮ�ĵ���̶ȴ�
���𰸡�C
��������
A.��ͼ���Կ�������Ũ�ȵ����������HA��HB��NaHCO3��Ӧ���ڿ�ʼһ��ʱ������ͬʱ��ų�CO2�����HB��HA�࣬˵��HA��Һ�е�c(H+)��HB��Һ�е�c(H+)С������HA<HB����A��ȷ��
B.��Ϊ����HA<HB������pH��Ϊ4��HA��HB��Һ��c(HA)![]() c(HB),�����к͵���NaOHʱ������HA��Һ���С����B��ȷ��
c(HB),�����к͵���NaOHʱ������HA��Һ���С����B��ȷ��
C.NaA��NaB��Ϊǿ�������Σ���Һ�Լ��ԣ�����pH��Ϊ9������ˮ�ĵ������ͬ����C����
D.Ũ�Ⱦ�Ϊ0.1mol/L��NaA��Һ��NaB��Һ��ȣ���������HA<HB������NaA��ˮ��̶ȴ�ˮ�ĵ���̶ȴ�D��ȷ��
�����ΪC��

��ϰ��ϵ�д�
 ���ɿ��õ�Ԫ����AB��ϵ�д�
���ɿ��õ�Ԫ����AB��ϵ�д� С�����ϵ�д�
С�����ϵ�д�
�����Ŀ