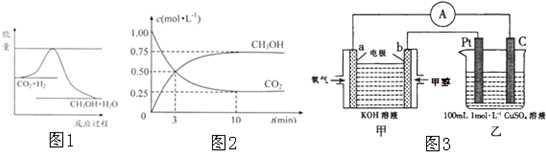
��Ŀ����
����������ЧӦ����Դ��ȱ�����⣬��ν��ʹ����е�CO2���������Կ������ã������˸������ձ����ӣ�Ŀǰ��ҵ����һ�ַ�������CO2����ȼ�ϼ״���һ�������·�����Ӧ��CO2��g��+3H2��g��?CH3OH��g��+H2O��g������H��0
��1���÷�Ӧƽ�ⳣ��K�ı���ʽΪ ���¶Ƚ��ͣ�ƽ�ⳣ��K ������������䡱��С������
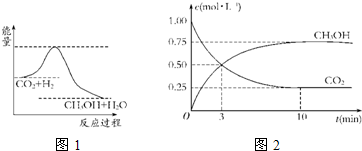
��2��Ϊ̽����Ӧԭ�����ֽ�������ʵ�飺�����Ϊ1L���ܱ������У�����1mol CO2��3mol H2�����CO2��CH3OH��g����Ũ����ʱ��仯��ͼ1��ʾ���ӷ�Ӧ��ʼ��ƽ�⣬������Ũ�ȱ仯��ʾ��ƽ����Ӧ����v��H2��Ϊ ��
��3�����д�ʩ����ʹn��CH3OH��/n��CO2��������� ��
A�������¶ȣ� B�����������C����H2O��g������ϵ�з��룻D���ٳ���1mol CO2��3mol H2��
E������He��g����ʹ��ϵ��ѹǿ����
��4����ͼ2��ʾ���ڼס����������зֱ�������ʵ���֮��Ϊ1��3 ��CO2��H2��ʹ�ס�����������ʼ�ݻ���ȣ�����ͬ�¶��·�����Ӧ����ά�ַ�Ӧ�������¶Ȳ��䣮������ȣ�ת���̶ȸ������ ����֪��������CO2��ת������ʱ��仯��ͼ����ͼ3��ʾ������ͼ3�л�����������CO2��ת������ʱ��仯��ͼ����˵����CO2Ϊԭ������ȼ�ϼ״����ŵ��� ��д��һ�����ɣ���
��һ�������£�FeS��KSP=2.5��10-18��H2S������Һ�ڸ��¶��£�[H+]��[S2-]���������¹�ϵ��[H+]2?[S2-]=1.0��10-21���ڸ��¶��£�������FeSͶ��H2S������Һ�У���ʹ��Һ��[Fe2+]�ﵽ1mol/L��Ӧ������Һ��pHΪ ���ö�����ʽ��ʾ������д��������̣�
��1���÷�Ӧƽ�ⳣ��K�ı���ʽΪ
��2��Ϊ̽����Ӧԭ�����ֽ�������ʵ�飺�����Ϊ1L���ܱ������У�����1mol CO2��3mol H2�����CO2��CH3OH��g����Ũ����ʱ��仯��ͼ1��ʾ���ӷ�Ӧ��ʼ��ƽ�⣬������Ũ�ȱ仯��ʾ��ƽ����Ӧ����v��H2��Ϊ
��3�����д�ʩ����ʹn��CH3OH��/n��CO2���������
A�������¶ȣ� B�����������C����H2O��g������ϵ�з��룻D���ٳ���1mol CO2��3mol H2��
E������He��g����ʹ��ϵ��ѹǿ����
��4����ͼ2��ʾ���ڼס����������зֱ�������ʵ���֮��Ϊ1��3 ��CO2��H2��ʹ�ס�����������ʼ�ݻ���ȣ�����ͬ�¶��·�����Ӧ����ά�ַ�Ӧ�������¶Ȳ��䣮������ȣ�ת���̶ȸ������

��һ�������£�FeS��KSP=2.5��10-18��H2S������Һ�ڸ��¶��£�[H+]��[S2-]���������¹�ϵ��[H+]2?[S2-]=1.0��10-21���ڸ��¶��£�������FeSͶ��H2S������Һ�У���ʹ��Һ��[Fe2+]�ﵽ1mol/L��Ӧ������Һ��pHΪ
��������1�����ݷ�Ӧ����ʽ��ƽ�ⳣ���ĺ�����д�����ʽ�������¶ȶ�ƽ���Ӱ�죬�ж�K�ı仯��
��2�����ݷ�Ӧ����v=
����v��CO2�����ٸ�������֮�ȵ��ڻ�ѧ������֮�ȼ���v��H2����
��3��Ҫʹ
����Ӧʹƽ��������Ӧ�����ƶ�������Ӱ��ƽ���ƶ������ط�����
��4���ڼס����������зֱ�������ʵ���֮��Ϊ1��3 ��CO2��H2��ʹ�ס�����������ʼ�ݻ���ȣ���ͼ��֪����ѹǿ���䣬�ҵ�������䣬�ɷ�Ӧ��֪�ҵ�ѹǿ���С�����ѹǿ�����ҵ�ѹǿ�����Լ�������CH4��ת���ʴ����ڼ���ѹǿ����Ӧ���ʿ죬�ﵽƽ���ʱ���С����CO2Ϊԭ������ȼ�ϼ״���ʹ���������ã������ڻ�����
����FeS��KSP���[Fe2+]�ﵽ1mol/Lʱ�������ӵ�Ũ�ȣ��ٸ���[H+]��[S2-]���ڵĹ�ϵ��������ӵ�Ũ�ȣ�
��2�����ݷ�Ӧ����v=
| ��c |
| ��t |
��3��Ҫʹ
| n(CH3OH) |
| n(CO2) |
��4���ڼס����������зֱ�������ʵ���֮��Ϊ1��3 ��CO2��H2��ʹ�ס�����������ʼ�ݻ���ȣ���ͼ��֪����ѹǿ���䣬�ҵ�������䣬�ɷ�Ӧ��֪�ҵ�ѹǿ���С�����ѹǿ�����ҵ�ѹǿ�����Լ�������CH4��ת���ʴ����ڼ���ѹǿ����Ӧ���ʿ죬�ﵽƽ���ʱ���С����CO2Ϊԭ������ȼ�ϼ״���ʹ���������ã������ڻ�����
����FeS��KSP���[Fe2+]�ﵽ1mol/Lʱ�������ӵ�Ũ�ȣ��ٸ���[H+]��[S2-]���ڵĹ�ϵ��������ӵ�Ũ�ȣ�
����⣺��1����֪CO2��g��+3H2��g��?CH3OH��g��+H2O��g������H��0������ƽ�ⳣ���ı���ʽΪ��K=
����֪�÷�Ӧ������Ϊ���ȷ������Խ����¶�ƽ�����ƣ���ƽ�ⳣ��K����
�ʴ�Ϊ��K=
������
��2���ӷ�Ӧ��ʼ��ƽ�⣬v��CO2��=
=0.075mol?L-1?min-1������֮�ȵ��ڻ�ѧ������֮�ȣ���v��H2��=3��0.075mol?L-1?min-1=0.225 mol?L-1?min-1���ʴ�Ϊ��0.225 mol?L-1?min-1��
��3��Ҫʹ
����Ӧʹƽ��������Ӧ�����ƶ���
A��������Ӧ���ȣ������¶�ƽ�����淴Ӧ�����ƶ�����
������A����
B�����������ƽ�ⲻ�ƶ�����n
���䣬��B����
C����H2O��g������ϵ�з��룬ƽ��������Ӧ�����ƶ�����
����C��ȷ��
D���ٳ���1mol CO2��3mol H2����Ч����ԭ����������С���һ�룬ѹǿ����ƽ��������Ӧ�����ƶ�����
����D��ȷ��
E������He��g����ʹ��ϵѹǿ�����Է�Ӧ������˵��Ũ��û�б仯��ƽ�ⲻ�ƶ���
���䣬��E����
�ʴ�Ϊ��CD��
��4���ڼס����������зֱ�������ʵ���֮��Ϊ1��3 ��CO2��H2��ʹ�ס�����������ʼ�ݻ���ȣ���ͼ��֪����ѹǿ���䣬�ҵ�������䣬�ɷ�Ӧ��֪�ҵ�ѹǿ���С�����ѹǿ�����ҵ�ѹǿ�����Լ�������CH4��ת���ʴ����ڼ���ѹǿ����ķ�Ӧ���ʿ죬�ﵽƽ���ʱ��̣����Լ�������CO2��ת������ʱ��仯��ͼ��Ϊ��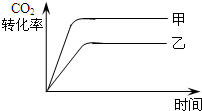 ����CO2Ϊԭ������ȼ�ϼ״�����ʹCO2���������ã������ڻ�����
����CO2Ϊԭ������ȼ�ϼ״�����ʹCO2���������ã������ڻ�����
�ʴ�Ϊ���ף� �����������ã������ڻ�����
�����������ã������ڻ�����
��Ҫʹ��Һ��[Fe2+]�ﵽ1mol/L����[S2-]=
=2.5��10-18mol/L����֪H2S������Һ�ڸ��¶��£�[H+]��[S2-]���������¹�ϵ��[H+]2?[S2-]=1.0��10-21��������Һ��[H+]=2��10-2mol/L����pH=2-lg2��
�ʴ�Ϊ��2-lg2��
| c(CH3OH)?c(H2O) |
| c(CO2)?c3(H2) |
�ʴ�Ϊ��K=
| c(CH3OH)?c(H2O) |
| c(CO2)?c3(H2) |
��2���ӷ�Ӧ��ʼ��ƽ�⣬v��CO2��=
| 1mol/L-0.25mol/L |
| 10min |
��3��Ҫʹ
| n(CH3OH) |
| n(CO2) |
A��������Ӧ���ȣ������¶�ƽ�����淴Ӧ�����ƶ�����
| n(CH3OH) |
| n(CO2) |
B�����������ƽ�ⲻ�ƶ�����n
| n(CH3OH) |
| n(CO2) |
C����H2O��g������ϵ�з��룬ƽ��������Ӧ�����ƶ�����
| n(CH3OH) |
| n(CO2) |
D���ٳ���1mol CO2��3mol H2����Ч����ԭ����������С���һ�룬ѹǿ����ƽ��������Ӧ�����ƶ�����
| n(CH3OH) |
| n(CO2) |
E������He��g����ʹ��ϵѹǿ�����Է�Ӧ������˵��Ũ��û�б仯��ƽ�ⲻ�ƶ���
| n(CH3OH) |
| n(CO2) |
�ʴ�Ϊ��CD��
��4���ڼס����������зֱ�������ʵ���֮��Ϊ1��3 ��CO2��H2��ʹ�ס�����������ʼ�ݻ���ȣ���ͼ��֪����ѹǿ���䣬�ҵ�������䣬�ɷ�Ӧ��֪�ҵ�ѹǿ���С�����ѹǿ�����ҵ�ѹǿ�����Լ�������CH4��ת���ʴ����ڼ���ѹǿ����ķ�Ӧ���ʿ죬�ﵽƽ���ʱ��̣����Լ�������CO2��ת������ʱ��仯��ͼ��Ϊ��
 ����CO2Ϊԭ������ȼ�ϼ״�����ʹCO2���������ã������ڻ�����
����CO2Ϊԭ������ȼ�ϼ״�����ʹCO2���������ã������ڻ������ʴ�Ϊ���ף�
 �����������ã������ڻ�����
�����������ã������ڻ�������Ҫʹ��Һ��[Fe2+]�ﵽ1mol/L����[S2-]=
| Ksp |
| [Fe2+] |
�ʴ�Ϊ��2-lg2��
���������⿼���Ϊ�ۺϣ��漰��ѧƽ��ͼ��ѧƽ������Լ�ƽ���ƶ����ܶȻ��������йؼ�������⣬��Ŀ�ѶȽϴ�ע�����ͼ���������ʵ�ƽ��Ũ��Ϊ������Ĺؼ���

��ϰ��ϵ�д�
 ��˼ά������ҵ��ټ��ִ�ѧ������ϵ�д�
��˼ά������ҵ��ټ��ִ�ѧ������ϵ�д� �����������Ż�ѧϰϵ�д�
�����������Ż�ѧϰϵ�д�
�����Ŀ
 ������CO2����ȼ�ϼ״���һ�������·�����Ӧ��CO2��g��+3H2��g��
������CO2����ȼ�ϼ״���һ�������·�����Ӧ��CO2��g��+3H2��g�� CH3OH��g��+H2O��g����������ͼ��ʾ�÷�Ӧ���й�������������λΪkJ?mol-1���ı仯��
CH3OH��g��+H2O��g����������ͼ��ʾ�÷�Ӧ���й�������������λΪkJ?mol-1���ı仯��