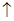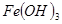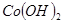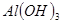��Ŀ����
�������������գ���Ŀǰ����ˮ���塱������Ҫ����֮һ���乤���������£�

��1���������ڱ���λ��________����__________�塣
��2����������������ữ�����Cl2�������ʣ�������___________________________��
��3�������������SO2�Ļ�ԭ�ԣ���Ӧ�����ӷ���ʽΪ__________________________��
��4����������������,�¶�Ӧ������80��90���¶ȹ�����Ͷ������������������ԭ��____________________________________________________��
��5���������������������õ�Һ������ˮ�Ļ������������ǵ�����ܶ����ܴ���ص���з��롣����������������______________________________________��
��6������١���֮��δֱ���á���Br2�ĺ�ˮ����������õ�Һ�壬���Ǿ�������������������SO2���ա�����������������������������������_______________________��

��1���������ڱ���λ��________����__________�塣
��2����������������ữ�����Cl2�������ʣ�������___________________________��
��3�������������SO2�Ļ�ԭ�ԣ���Ӧ�����ӷ���ʽΪ__________________________��
��4����������������,�¶�Ӧ������80��90���¶ȹ�����Ͷ������������������ԭ��____________________________________________________��
��5���������������������õ�Һ������ˮ�Ļ������������ǵ�����ܶ����ܴ���ص���з��롣����������������______________________________________��
��6������١���֮��δֱ���á���Br2�ĺ�ˮ����������õ�Һ�壬���Ǿ�������������������SO2���ա�����������������������������������_______________________��
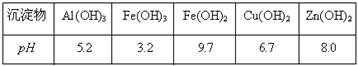
(1)4��VIIA����2�֣� (2)�ữ������Cl2��Br2��ˮ��Ӧ(3��) (3)Br2+SO2+2H2O=4H++2Br-+SO42-(2��)
(4)�¶ȹ��ߣ���������ˮ��������������ˮ�������ӣ��¶ȹ��ͣ��岻����ȫ�����������ʵ͡���3�֣�
(5)��Һ©��(2��) (6)������������SO2���ա��Ȼ����Ĺ���ʵ������һ��Br2��Ũ�����̣���ֱ������Br2��ˮ���Ч�ʸ��ߣ�������Դ�٣��ɱ����͡���3�֣�
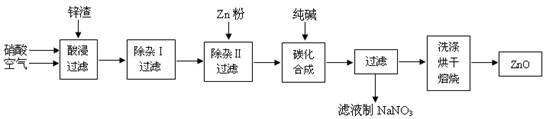
���������������һ�����������⡣����ѡ��2���ݡ���ѧ�뼼����һ�顣��ˮ�к���Ca2+��Mg2+��Na+�������ӣ���Ȼ������Cl-��SO42-��Br-�������ӡ���һ���Ӻ�ˮ����ȡ���κ�ʣ����Һ��֮Ϊ��±���ټ������ữ��������������Ŀ���ǽ�Br-����ΪBr2����ͨ������Br2�������õ���Br2�Ŀ�����SO2���յõ�����Һ��Br2+SO2+2H2O=H2SO4+2HBr����������Һ�ֽ���������õ���ˮ�����ٽ�������õ�Br2�������ٽ���������������յõ�Һ�塣
��1���������ڱ���λ��4����VIIA�塣ע����д�������ڼȿ���д����������Ҳ����д���֡�������ֻ�����������֡�������A��ʾ��������B��ʾ��
��2����������������ữ�����Cl2�������ʣ��ữ������Cl2��Br2��ˮ��Ӧ��
��3�������������SO2�Ļ�ԭ�ԣ���Ӧ�����ӷ���ʽΪBr2+SO2+2H2O=4H++2Br-+SO42-��
��4����������������,�¶�Ӧ������80��90���¶ȹ�����Ͷ��������������¶ȹ���ʹ��ˮ�����������¶�̫����ӷ������������ʵ͡�
��5���������������������õ�Һ������ˮ�Ļ������������ǵ�����ܶ����ܴ���ص㣬���÷�Һ�������õ������Ƿ�Һ©������Һ�������е�������Һ���ܶȲ�ͬ�������ܣ���һ������������Һ�����ܽ�������Բ��졣���������CCl4��Br2�������л��ܼ�������ˮ���ܽ�Ȳ���
��6������١���֮��δֱ���á���Br2�ĺ�ˮ����������õ�Һ�壬���Ǿ�������������������SO2���ա�����������������������������SO2���ա��Ȼ����Ĺ���ʵ������һ��Br2��Ũ�����̣���ֱ������Br2��ˮ���Ч�ʸ��ߣ�������Դ�٣��ɱ����͡�

��ϰ��ϵ�д�
 ͨ��ѧ��Ĭд����ϵ�д�
ͨ��ѧ��Ĭд����ϵ�д� ���ƽ̸�������ѡ����ĩ���100��ϵ�д�
���ƽ̸�������ѡ����ĩ���100��ϵ�д�
�����Ŀ



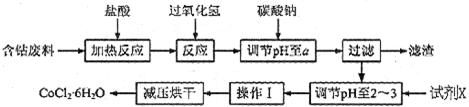
 H2O�ڼ���ʱ����ʧˮ�������ֲ�ͬ����ɫ��ʵ����������Ƴɱ�ɫ�轺�����Ը��ﲢ��֤ˮ�ݡ��Ժ��ܷ��ϣ�������Fe��Al�����ʣ���ȡCoCl2
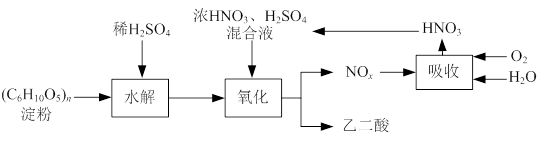
H2O�ڼ���ʱ����ʧˮ�������ֲ�ͬ����ɫ��ʵ����������Ƴɱ�ɫ�轺�����Ը��ﲢ��֤ˮ�ݡ��Ժ��ܷ��ϣ�������Fe��Al�����ʣ���ȡCoCl2 H2O��һ���¹�����������ͼ��
H2O��һ���¹�����������ͼ��