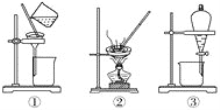
��Ŀ����
����Ŀ���ڳ��³�ѹ�£���֪��
��4Fe(s)��3O2(g)= 2Fe2O3(s)����H1
��4Al(s)��3O2(g)= 2Al2O3(s)����H2
��2Al(s)��Fe2O3(s)= Al2O3(s)��2Fe(s)����H3
��H3�릤H1����H2֮��Ĺ�ϵ��ȷ����
A. ��H3��![]() (��H1����H2) B. ��H3��(��H2����H1)
(��H1����H2) B. ��H3��(��H2����H1)
C. ��H3��2(��H1����H2) D. ��H3��![]() (��H2����H1)
(��H2����H1)
���𰸡�D
��������
���ݸ�˹����������֪���Ȼ�ѧ����ʽ������Ӧ����ֵ���мӼ�������Ŀ���Ȼ�ѧ����ʽ����Ӧ��Ҳ������Ӧ����ֵ���мӼ����ݴ��ж���
��֪����4Fe(s)��3O2(g)= 2Fe2O3(s)����H1
��4Al(s)��3O2(g)= 2Al2O3(s)����H2
��2Al(s)��Fe2O3(s)= Al2O3(s)��2Fe(s)����H3
�ɸ�˹���ɿ�֪����-�ٵ�4Al��s��+2Fe2O3��s��=2Al2O3��s��+4Fe��s����H=��H2-��H1����2Al(s)��Fe2O3(s)= Al2O3(s)��2Fe(s)����H3=![]() ����H2-��H1������ѡD��
����H2-��H1������ѡD��

��ϰ��ϵ�д�
�����Ŀ