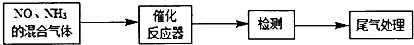
��Ŀ����
11����ͼ�Ƕ����ѽ��ʵ�����̣�����ʾ��������һ���������ѽ�Ŀ��ܷ���ʽΪ��C4H10$��_{��}^{����}$C2H6+C2H4��C4H10$��_{��}^{����}$CH4+C3H6��
���Ӻ�װ�ú�����е�ʵ������У��ٸ�D��Gװ�ü��ȣ��ڼ������װ�õ������ԣ����ų�װ���еĿ����ȡ��E
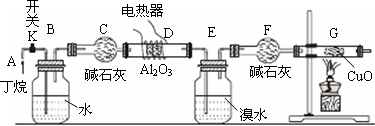
��1���������������Ⱥ�˳�������Ǣڢۢ٣�
��2����Ҫ˵���ſ����ķ�����K��ʹ����������������װ��
��3��д������������ͭ��Ӧ�Ļ�ѧ����ʽCH4+4CuO$��_{��}^{Al_{2}O_{3}}$CO2+2H2O+4Cu
��4������Eװ���еĻ�����ˮ���������ٰ���������ʵ�飺
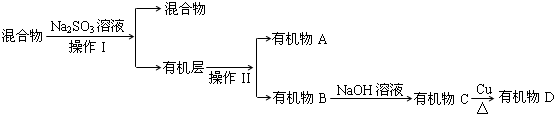
�ٷ��������͢�����Ʒֱ��ǣ����Һ��������Na2SO3��Һ�������ǣ������ӷ���ʽ��ʾ��SO22-+Br2+H2O=SO42-+2Br-+2H+��
����֪B��̼ԭ��������A��̼ԭ��������д��B�Ľṹ��ʽCH2BrCHBrCH3��
��5���ٶ�������ȫ�ѽ��E+F��װ�õ��������ȷ�Ӧǰ������0.7g��Gװ�õ�����������1.76g��������ѽ�����м������������ʵ���֮��n ��CH4����n ��C2H6��=1��1���ٶ�����D��Gװ���е���������ȫ��Ӧ��
���� ��K������ͨ��B��Bװ���Ǹ������ݿ����������٣�Cװ�ø��ﶡ�飬�������������������¶��鷢���ѽⷴӦ����ϩ����������E����ˮ����ϩ����F����������G��������Cu�ڼ��������·���������ԭ��Ӧ����Cu��
��1��Ӧ�ȼ��������ԣ��ϳ��ڲ����壬�ټ��ȣ�
��2����K��ʹ����������������װ�ã�
��3�����������£������CuO����������ԭ��Ӧ���ɶ�����̼��Cu��ˮ��
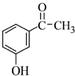
��4��������к����塢ˮ��������������������ƣ��������Ʊ����������������ƣ�ͬʱ����NaBr���Ӷ���ȥ�壬Ȼ����÷�Һ�������룬���л�����з���õ��л���A���л���B�����л����м���NaOH��Һ���õ��л���C��C�ܷ���������Ӧ����B����ˮ�ⷴӦ����CΪ����C���������õ�ȩD��
��5��E��F���յ���ϩ����G���ٵ�����������ͭ�е���Ԫ��������������ѽ��У�������ϩ�����ʵ�������������ʵ�����ȣ�����ͱ�ϩ�����ʵ�����ȣ��ٽ��ԭ���غ����������������ʵ���֮�ȣ�
��� �⣺��K������ͨ��B��Bװ���Ǹ������ݿ����������٣�Cװ�ø��ﶡ�飬�������������������¶��鷢���ѽⷴӦ����ϩ����������E����ˮ����ϩ����F����������G��������Cu�ڼ��������·���������ԭ��Ӧ����Cu��
��1��Ӧ�ȼ��������ԣ��ϳ��ڲ����壬�ٸ�D��Gװ�ü��ȣ�
�ʴ�Ϊ���ڢۢ٣�
��2����K��ʹ����������������װ�ã��Ӷ��������ų����ʴ�Ϊ����K��ʹ����������������װ�ã�
��3�������������������������£����������ͭ��Ӧ���ɶ�����̼��ˮ��ͭ����Ӧ����ʽΪ��CH4+4CuO$��_{��}^{Al_{2}O_{3}}$CO2+2H2O+4Cu��
�ʴ�Ϊ��CH4+4CuO$��_{��}^{Al_{2}O_{3}}$CO2+2H2O+4Cu��
��4��������к����塢ˮ��������������������ƣ��������Ʊ����������������ƣ�ͬʱ����NaBr���Ӷ���ȥ�壬Ȼ����÷�Һ�������룬���л�����з���õ��л���A���л���B�����л����м���NaOH��Һ���õ��л���C��C�ܷ���������Ӧ����B����ˮ�ⷴӦ����CΪ����C���������õ�ȩD��
��ͨ�����Ϸ���֪�����������͢�����Ʒֱ��ǣ����Һ���������������ƾ��л�ԭ�ԣ��ܺ�ǿ�����������巴Ӧ����ȥ�壬���ӷ���ʽΪ��SO22-+Br2+H2O=SO42-+2Br-+2H+��
�ʴ�Ϊ����Һ������SO22-+Br2+H2O=SO42-+2Br-+2H+��
����֪B��̼ԭ��������A��̼ԭ������˵��B��̼ԭ�Ӹ�����3��A��̼ԭ�Ӹ�����2��BΪ1��2-������飬B�Ľṹ��ʽCH2BrCHBrCH3��
�ʴ�Ϊ��CH2BrCHBrCH3��
��5��������ѽ������ɵ���ϩ����������ʵ�����ȣ����ɵļ���ͱ�ϩ�����ʵ�����ȣ�
E��F���յ���ϩ����G���ٵ�����������ͭ�е���Ԫ��������
��XΪC2H4�����ʵ�����yΪC3H6�����ʵ�����������ͼ�������ʵ����ֱ���x��y��
28x+42y=0.7g
����ͼ��������ͭ��Ӧ��Ҫ����ԭ�ӵ����ʵ���Ϊ
2��2x+y��+$\frac{6x+2y}{2}$=$\frac{1.76}{16}$��
��ã�x=y=0.07mol
�ʴ�Ϊ��1��1��
���� �����Զ����ѽ�Ϊ���忼��ʵ��������������㡢���ʵķ�����ᴿ����ȷ����ͼ�и���װ�õ����á����ʷ�����ᴿ������ѡȡ��֪ʶ�㼴�ɽ���ѵ��ǣ�5������㣬��ȷ�������ӵ������������ٵ����ֱ���ʲô�����ǽ⣨5���Ĺؼ�����Ŀ�Ѷ��еȣ�

 ����������ϵ�д�
����������ϵ�д�| A�� | �� | B�� | ���Ը������ | C�� | ��ˮ | D�� | ����������ͭ |
| A�� | 46gNO2��N2O4�Ļ������У�����ԭ����Ŀ��NA | |
| B�� | 1.0L 0.1mol/L��NaF��Һ�У���F-������Ŀ��0.1NA | |
| C�� | 11.2L��H2�У����еĵ�����Ŀ��NA | |
| D�� | 1molFeCl3����ˮ��ȫ��Ӧת��Ϊ�����������壬���н������ӵ���Ŀ��NA |
| A�� | �ۢܢݢ� | B�� | �ۢܢ� | C�� | �ܢ� | D�� | �ܢޢ� |
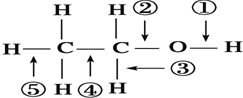
| A�� | �Ҵ����Ʒ�Ӧ�����ٶ��� | |
| B�� | �Ҵ���Ag����������O2��Ӧ�����٢۶��� | |
| C�� | �Ҵ���ȫȼ��ʱ��ֻ�Т٢ڼ����� | |
| D�� | �Ҵ��������Ũ���Ṳ�����������������ٶ��� |
| A�� |  | B�� | CH3CH2CH2OH | C�� | 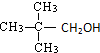 | D�� |  |
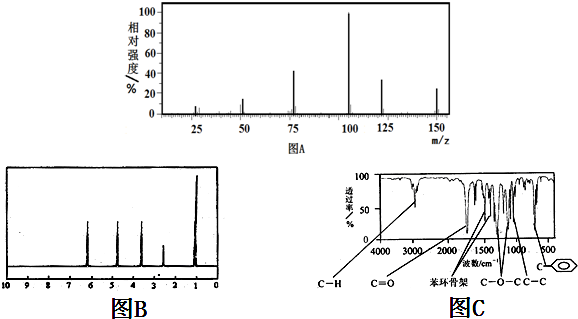
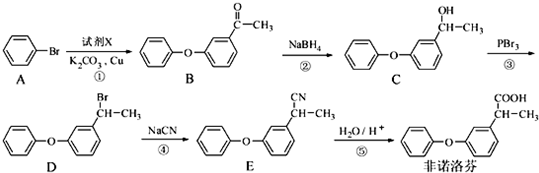
 ��
��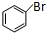 +2NaOH$\stackrel{һ������}{��}$
+2NaOH$\stackrel{һ������}{��}$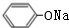 +NaBr+H2O��
+NaBr+H2O��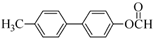 ��
��