��Ŀ����
17���뽫��ȷѡ���������ڶ�Ӧ�ĺ����ϣ�ÿС�ʿ�����1������ѡ��������⣩A������������ܶȲ��� B�����������ƽ������������
C������������������� D�������е�ѹǿ����
E������������ɫ���� F���������������
��1��һ���¶��£������ܱյ������г���һ������C��s����H2O��g����������Ӧ��
C��s��+H2O��g��?H2��g��+CO��g��������ѡ��١����У�����֤���÷�Ӧ�Ѿ��ﵽƽ��״̬����ABCD��
��2��һ���¶ȣ������ܱյ������з�����Ӧ��I2��g��+H2��g��?2HI��g��������ѡ��١����У�����֤���÷�Ӧ�Ѿ��ﵽƽ��״̬����E��
��3��һ���¶ȣ���ѹ�ܱյ������з�����Ӧ��2SO2��g��+O2��g��?2SO3��g��������ѡ��١����У�����֤���÷�Ӧ�Ѿ��ﵽƽ��״̬����ABF��
���� ���ݻ�ѧƽ��״̬��������𣬵���Ӧ�ﵽƽ��״̬ʱ�����淴Ӧ������ȣ������ʵ�Ũ�ȡ��ٷֺ������䣬�Լ��ɴ�������һЩ��Ҳ�������仯������ʱҪע�⣬ѡ���жϵ������������ŷ�Ӧ�Ľ��з����仯�������������ɱ仯����ֵʱ��˵�����淴Ӧ����ƽ��״̬��
��� �⣺��1��һ���¶��£������ܱյ������г���һ������C��s����H2O��g����������Ӧ��C��s��+H2O��g��?H2��g��+CO��g��������ѡ��١����У�����֤���÷�Ӧ�Ѿ��ﵽƽ��״̬��������������ܶȲ��䡢���������ƽ�����������䡢����������������䡢�����е�ѹǿ���䶼˵����ƽ��״̬��
�ʴ�Ϊ��ABCD��
��2��һ���¶ȣ������ܱյ������з�����Ӧ��I2��g��+H2��g��?2HI��g��������ѡ��١����У�����֤���÷�Ӧ�Ѿ��ﵽƽ��״̬���ǻ���������ɫ���䣬��ѡE��
��3��һ���¶ȣ���ѹ�ܱյ������з�����Ӧ��2SO2��g��+O2��g��?2SO3��g��������ѡ��١����У�����֤���÷�Ӧ�Ѿ��ﵽƽ��״̬��������������ܶȲ��䡢���������ƽ�����������䡢������������䣬��˵����ƽ��״̬���ʴ�Ϊ��ABF��
���� ���⿼���˻�ѧƽ��״̬���жϣ�ע���Ӧ�ﵽƽ��״̬ʱ�����淴Ӧ������ȣ�ע�����֪ʶ�����գ�

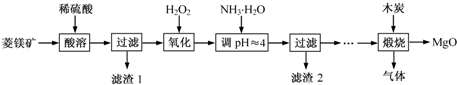
��ʽһ��һ�ֺ�ͭ�Ŀ�ʯ�����ȸʯ��ۣ�����ͭ��̬ΪCuCO3•Cu��OH��2��CuSiO3•2H2O������SiO2��FeCO3��Fe2O3��Al2O3�����ʣ��������ֿ�ʯΪԭ����ȡ����ͭ�Ĺ���������ͼ��
��ش��������⣺
��1����ɲ������ϡ������CuSiO3•2H2O������Ӧ�Ļ�ѧ����ʽCuSiO3•2H2O+H2SO4=CuSO4+H4SiO4+H2O��
��2������ڵ�����ҺpHѡ�õ�����Լ���A
A��CuOB��MgO C��FeCO3D NH3•H2O
��3���й��������↑ʼ��������ȫ������pH���±���
| �������� | Al��OH��3 | Fe��OH��3 | Fe��OH��2 | Cu��OH��2 |
| ��ʼ������pH | 3.3 | 1.5 | 6.5 | 4.2 |
| ������ȫ��pH | 5.2 | 3.7 | 9.7 | 6.7 |
��4����ҺBͨ������Ũ���������Ũ��Ϊԭ����һ�룩����ȴ�ᾧ���Եõ�CuSO4•5H2O���壮ijͬѧ��Ϊ������������������������������������������ݶԸ�ͬѧ�Ĺ۵��������ۣ���֪�����£�Al2��SO4��3������Һ��C��Al3+��=2.25mol•L-1��Ksp[Al��OH��3]=3.2��10-34���������ȷ��������
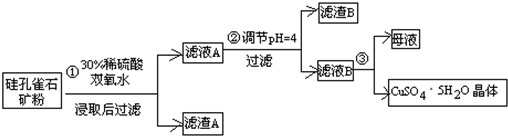
��ʽ�����Ի�ͭ��Ϊԭ�ϣ���ȡ����ͭ������ͭ�Ĺ���������ʾ��
����ͭ����Ҫ�ɷ�ΪCuFeS2����������CaO��MgO��Al2O3������
������ͼװ�ý��е绯ѧ����ʵ�飬����ѡ��ͭ��ۼ�����������������ٽ��裬ʹ����ܽ⣮��������ͨ��������������������������һ��ʱ���ȡ��������Һ�������м����л���ȡ����RH��������Ӧ��
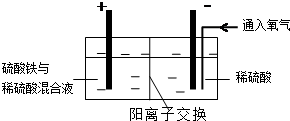
2RH���л��ࣩ+Cu2+��ˮ�ࣩ?R2Cu���л��ࣩ+2H+��ˮ�ࣩ������л��࣬�����м���һ��Ũ�ȵ����ᣬʹCu2+����������
�����������ͭ��Һ�Ƶý���ͭ��
��5����ͭ��ۼ��������������ἰ��������Ҫ�������·�Ӧ��
CuFeS2+4H+=Cu2++Fe2++2H2S 2Fe3++H2S=2Fe2++S��+2H+
�������У�������Fe3+��Ũ�Ȼ������ֲ��䣬ԭ����Fe2+-e-=Fe3+���õ缫��Ӧʽ��ʾ����
��6����������л����м���һ��Ũ�ȵ����ᣬCu2+����������ԭ��������H+Ũ�ȣ�ʹƽ��2RH���л��ࣩ+Cu2+��ˮ�ࣩ?R2Cu���л��ࣩ+2H+��ˮ�ࣩ�����ƶ���Cu2+����ˮ�����������
��7��������������0.1mol CuSO4��Һ������ͭ3.2g����ʱ��Һ������Ũ���ɴ�С��˳����c��H+����c��SO42-����c��Cu2+����c��OH-����
 2013���������������Ű�ҹ��ж������������У�����β����ȼúβ������ɿ�����Ⱦ��ԭ��֮һ��
2013���������������Ű�ҹ��ж������������У�����β����ȼúβ������ɿ�����Ⱦ��ԭ��֮һ����1��CO2�Ǵ����к�����ߵ�һ���������壬���ƺ�����CO2�ǽ������ЧӦ����Ч;����Ŀǰ����CO2���ϳɶ�������ȡ���˽ϴ�Ľ�չ���仯ѧ��Ӧ�ǣ�
2CO2��g��+6H2��g��?CH3OCH3��g��+3H2O��g����H��0��
��д���÷�Ӧ��ƽ�ⳣ������ʽ$\frac{c��C{H}_{3}OC{H}_{3}����{c}^{3}��{H}_{2}O��}{{c}^{2}��C{O}_{2}����{c}^{6}��{H}_{2}��}$��
���жϸ÷�Ӧ��һ�������£�����㶨���ܱ��������Ƿ�ﵽ��ѧƽ��״̬��������BD��
A���������ܶȲ��� B����λʱ��������2molCO2��ͬʱ����1mol������
C��v��CO2����v��H2��=1��3 D��������ѹǿ���ֲ���
��2������β����������Ҫԭ��Ϊ��2NO��g��+2CO��g�� $\stackrel{����}{?}$2CO2��g��+N2 ��g��
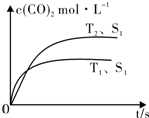
���ܱ������з����÷�Ӧʱ��c��CO2�����¶ȣ�T���������ı������S����ʱ�䣨t���ı仯���ߣ���ͼ��ʾ���ݴ��жϣ�
�ٸ÷�Ӧ�ġ�H��0��ѡ�����������������
�ڵ��������������һ��ʱ�����������������ѧ��Ӧ���ʣ��������ı����S1��S2����ͼ�л���c��CO2����T2��S2�����´ﵽƽ������еı仯���ߣ�
��3����֪��CO��g��+2H2��g��?CH3OH��g����H=-a kJ•mol-1��
�پ��ⶨ��ͬ�¶��¸÷�Ӧ��ƽ�ⳣ�����£�
| �¶ȣ��棩 | 250 | 300 | 350 |
| K | 2.041 | 0.270 | 0.012 |
��ij�¶��£�������̶���2L���ܱ������н�1mol CO��2mol H2��ϣ���ò�ͬʱ�̵ķ�Ӧǰ��ѹǿ��ϵ���£�
| ʱ�䣨min�� | 5 | 10 | 15 | 20 | 25 | 30 |
| ѹǿ�ȣ�P��/Pǰ�� | 0.98 | 0.90 | 0.80 | 0.70 | 0.70 | 0.70 |
��4��Һ����Ϊһ��DZ�ڵ��������ȼ����Խ��Խ���о���Ա���ӣ����ڰ�ȫ�ԡ��۸�ȷ���ϻ�ʯȼ�Ϻ���ȼ�����Žϴ�����ƣ�����ȼ��ʵ������صķ�Ӧ�У�
4NH3��g��+3O2��g��=2N2��g��+6H2O��l����H1 ��
4NH3��g��+5O2��g��=4NO��g��+6H2O��l����H2 ��
4NH3��g��+6NO��g��=5N2��g��+6H2O��l����H3 ��
��д������������Ӧ�С�H1����H2����H3����֮���ϵ�ı���ʽ����H1=$\frac{3��{H}_{2}+2��{H}_{3}}{5}$��
��5������Simons�ȿ�ѧ�ҷ�����ʹNH3ֱ������ȼ�ϵ�صķ�������װ��Ϊ�ò���Ϊ�缫������KOH�������Һ�У����ط�ӦΪ 4NH3+3O2=2N2+6H2O��д����ȼ�ϵ�ص�������ӦʽO2+2H2O+4e-=4OH-��
| A�� | 22.4�� | B�� | 44.8�� | C�� | 11.2�� | D�� | 4.48�� |
| A�� | 1molNO��������30g | |
| B�� | ��״���£�1molH2O�������22.4L | |
| C�� | 17gNH3���е���ԭ������Ϊ6.02��1023 | |
| D�� | 100mL0.1mol/L Na2CO3��Һ�У�Na+�����ʵ���Ϊ 0.01mol |
| A�� | ���������ӵĽṹ��ʽ�� | B�� | ����ı���ģ�ͣ�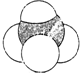 | ||
| C�� | ���ĵ���ʽ��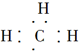 | D�� | �����ǵ�ʵ��ʽ��CH2O |