��Ŀ����
��14�֣�CO2��ת�����л���ʵ��̼ѭ����
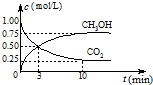
��1�������Ϊ1 L���ܱ������У�����1 mol CO2��3 mol H2��һ�������·�����Ӧ�� CO2(g)+3H2(g) CH3OH(g)+H2O(g) ��H=��49.0 mol��L-1�����CO2��CH3OH(g)��Ũ����ʱ��仯��ͼ��ʾ��
CH3OH(g)+H2O(g) ��H=��49.0 mol��L-1�����CO2��CH3OH(g)��Ũ����ʱ��仯��ͼ��ʾ��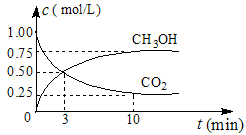
�ٴ�0 min��10 min��v(H2)= mol��(L��min)-1��
����˵��������Ӧ�ﵽƽ��״̬���� ��ѡ���ţ���
A����Ӧ��CO2��CH3OH�����ʵ���Ũ��֮��Ϊ1�U1����ͼ�н���㣩
B�������������ѹǿ����ʱ��ı仯���仯
C����λʱ����ÿ����3 mol H2��ͬʱ����1 mol H2O
D��CO2����������ڻ�������б��ֲ���
�����д�ʩ����ʹn (CH3OH)/n (CO2)������� ��ѡ���ţ���
A����H2O(g)����ϵ�з��� B�����º��ݳ���He
C�����º�ѹ����He D�����º����ٳ���1 mol CO2��3 mol H2
��2���ݱ�����һ���������ɶ�����̼�������ϳɶ������ѳ�Ϊ��ʵ��
2CO2(g)+6H2(g)  CH3OCH3(g)+3H2O(g)
CH3OCH3(g)+3H2O(g)
��һ��ѹǿ�£���÷�Ӧ��ʵ���������±���
�����������ݻش��������⣺
�ٷ�Ӧ���¶����ߣ�Kֵ �����������С�����䡱����
�������̼��[n��H2��/n��CO2��], Kֵ �����������С�����䡱����
��3��800��ʱ��C(s)��CO2(g) 2CO(g)��ƽ�ⳣ��K��1.64����ͬ�����²��c(CO)��0.20 mol��L��1��c(CO2)��0.05 mol��L��1����ʱ��Ӧ�� ����������桱��������С�
2CO(g)��ƽ�ⳣ��K��1.64����ͬ�����²��c(CO)��0.20 mol��L��1��c(CO2)��0.05 mol��L��1����ʱ��Ӧ�� ����������桱��������С�
��4�����ܱ�������ͨ��1mol H2��1mol CO2����H2(g)+CO2(g)  CO(g)+H2O(g) ��H> 0��Ӧ������Ӧ�ﵽƽ�����������������ʱ���������¶ȣ�������ͼ�л�����(v��)����(v��)��Ӧ������ʱ��t�仯��ʾ��ͼ��
CO(g)+H2O(g) ��H> 0��Ӧ������Ӧ�ﵽƽ�����������������ʱ���������¶ȣ�������ͼ�л�����(v��)����(v��)��Ӧ������ʱ��t�仯��ʾ��ͼ��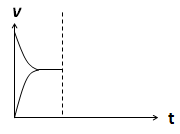
��1����0.225 ��BD ��AD ��2����С ����
��3���� (4) 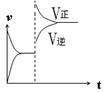
����

 ȫ�ŵ�����Ԫ�ƻ�ϵ�д�
ȫ�ŵ�����Ԫ�ƻ�ϵ�д�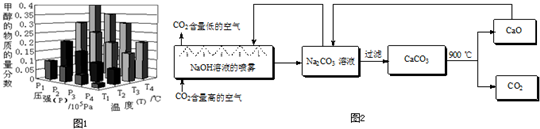

 CO2��SO2��NOx�ǶԻ���Ӱ��ϴ�����壬���ƺ�����CO2��SO2��NOx�ǽ������ЧӦ����������⻯ѧ��������Ч;����
CO2��SO2��NOx�ǶԻ���Ӱ��ϴ�����壬���ƺ�����CO2��SO2��NOx�ǽ������ЧӦ����������⻯ѧ��������Ч;���� CO+3H2
CO+3H2