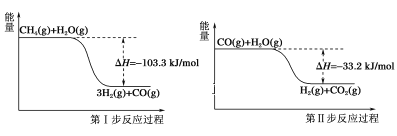
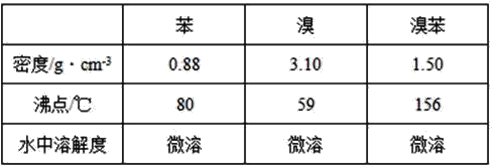
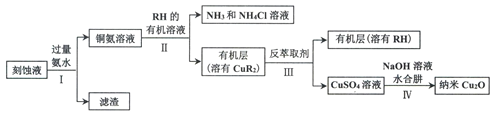
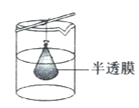
��Ŀ����
����Ŀ��ij����![]() ��������FeO��
��������FeO��![]() ��MgO��
��MgO��![]() �����ʣ��ô˷�����ȡ
�����ʣ��ô˷�����ȡ![]() �Ĺ���������ͼ1��
�Ĺ���������ͼ1��
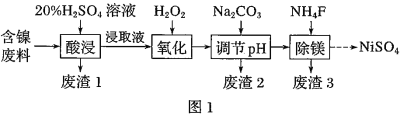
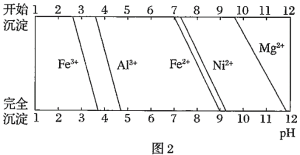
��֪�����йؽ���������������������������pH��ͼ��
��![]() ʱ��
ʱ��![]() �ĵ��볣��
�ĵ��볣��![]() �ĵ��볣��
�ĵ��볣��![]() ��
��![]()
![]() ��
��
(1)��![]() ������Һ��pH��5���õ�����2����Ҫ�ɷ���______
������Һ��pH��5���õ�����2����Ҫ�ɷ���______![]() �ѧʽ
�ѧʽ![]() ��
��
(2)![]() ���뱥��
���뱥��![]() ��Һ��Ӧ����
��Һ��Ӧ����![]() �����û�ѧƽ���ƶ�ԭ������
�����û�ѧƽ���ƶ�ԭ������![]() �ñ�Ҫ�����ֺ����ӷ���ʽ�ش�
�ñ�Ҫ�����ֺ����ӷ���ʽ�ش�![]() ______��
______��
(3)![]() ʱ��
ʱ��![]() ��NaF��Һ��
��NaF��Һ��![]() ______
______![]() �г�����ʽ����
�г�����ʽ����![]() ��Һ��______
��Һ��______![]() ������������������������������
������������������������������![]() ��
��
(4)��֪����ǰ��Һ��![]() ������þ�ʴﵽ
������þ�ʴﵽ![]() ʱ����Һ��
ʱ����Һ��![]() ______
______![]() ��
��
(5)��NaOH��Һ����NaClO��![]() ��Ӧ�ɵ�
��Ӧ�ɵ�![]() ����ѧ����ʽΪ____________��
����ѧ����ʽΪ____________��![]() ������������Ͻ�����������Ե��
������������Ͻ�����������Ե��![]() ��Һ
��Һ![]() ������ԭ��Ϊ��
������ԭ��Ϊ��![]() �������ĵ缫��Ӧʽ��______��
�������ĵ缫��Ӧʽ��______��
���𰸡�![]() ��
��![]() �Ȼ��ˮ�����������һˮ�ϰ���
�Ȼ��ˮ�����������һˮ�ϰ���![]() ��þ�������ӷ�Ӧ����������������Ũ�ȼ�С���ٽ�ƽ��������У����ɵ�һˮ�ϰ��ֽ����ɰ�����
��þ�������ӷ�Ӧ����������������Ũ�ȼ�С���ٽ�ƽ��������У����ɵ�һˮ�ϰ��ֽ����ɰ�����![]()
![]() ����
���� ![]()
![]()
![]()
��������
ijNiO�ķ�������FeO��![]() ��MgO��
��MgO��![]() �����ʣ�����ϡ�����ܽ����˵õ�����1Ϊ
�����ʣ�����ϡ�����ܽ����˵õ�����1Ϊ![]() ����ҺΪ
����ҺΪ![]() ��
��![]() ��
��![]() ��
��![]() �������������������������Ϊ�����ӣ��ټ���̼������Һ������ҺpH��ʹ�����ӣ�������ȫ�����������˵õ�����2Ϊ��������������������������Һ�м���
�������������������������Ϊ�����ӣ��ټ���̼������Һ������ҺpH��ʹ�����ӣ�������ȫ�����������˵õ�����2Ϊ��������������������������Һ�м���![]() ����
����![]() �����ɳ�������3Ϊ
�����ɳ�������3Ϊ![]() �����˵õ�����Һ����Һ�л��
�����˵õ�����Һ����Һ�л��![]() ����ķ�����ͨ������Ũ������ȴ�ᾧ������ϴ�ӣ�����õ����壬ʧȥ�ᾧˮ�õ����������ݴ˷�����
����ķ�����ͨ������Ũ������ȴ�ᾧ������ϴ�ӣ�����õ����壬ʧȥ�ᾧˮ�õ����������ݴ˷�����
ijNiO�ķ�������FeO��![]() ��MgO��
��MgO��![]() �����ʣ�����ϡ�����ܽ����˵õ�����1Ϊ
�����ʣ�����ϡ�����ܽ����˵õ�����1Ϊ![]() ����ҺΪ
����ҺΪ![]() ��
��![]() ��
��![]() ��
��![]() �������������������������Ϊ�����ӣ��ټ���̼������Һ������ҺpH��ʹ�����ӣ�������ȫ�����������˵õ�����2Ϊ��������������������������Һ�м���
�������������������������Ϊ�����ӣ��ټ���̼������Һ������ҺpH��ʹ�����ӣ�������ȫ�����������˵õ�����2Ϊ��������������������������Һ�м���![]() ����
����![]() �����ɳ�������3Ϊ
�����ɳ�������3Ϊ![]() �����˵õ�����Һ����Һ�л��
�����˵õ�����Һ����Һ�л��![]() ����ķ�����ͨ������Ũ������ȴ�ᾧ������ϴ�ӣ�����õ����壬ʧȥ�ᾧˮ�õ���������
����ķ�����ͨ������Ũ������ȴ�ᾧ������ϴ�ӣ�����õ����壬ʧȥ�ᾧˮ�õ���������
![]() ����̼������Һ������ҺpH��ʹ�����ӣ�������ȫ�����������������������������������õ�����2����Ҫ�ɷ���
����̼������Һ������ҺpH��ʹ�����ӣ�������ȫ�����������������������������������õ�����2����Ҫ�ɷ���![]() ��
��![]() ��
��
�ʴ�Ϊ��![]() ��
��![]() ��
��
![]() ���뱥��
���뱥��![]() ��Һ��Ӧ����
��Һ��Ӧ����![]() ���Ȼ��ˮ�����������һˮ�ϰ���
���Ȼ��ˮ�����������һˮ�ϰ���![]() ��þ�������ӷ�Ӧ����������������Ũ�ȼ�С���ٽ�ƽ��������У����ɵ�һˮ�ϰ��ֽ����ɰ�����
��þ�������ӷ�Ӧ����������������Ũ�ȼ�С���ٽ�ƽ��������У����ɵ�һˮ�ϰ��ֽ����ɰ�����![]() ��
��
�ʴ�Ϊ���Ȼ��ˮ�����������һˮ�ϰ���![]() ��þ�������ӷ�Ӧ����������������Ũ�ȼ�С���ٽ�ƽ��������У����ɵ�һˮ�ϰ��ֽ����ɰ�����
��þ�������ӷ�Ӧ����������������Ũ�ȼ�С���ٽ�ƽ��������У����ɵ�һˮ�ϰ��ֽ����ɰ�����![]() ��
��
![]() ʱ��
ʱ��![]() ��NaF��Һ��
��NaF��Һ��![]() ����ϵ���ƽ�ⳣ��
����ϵ���ƽ�ⳣ��![]() ��
�� ��ˮ��ƽ��ʱ
��ˮ��ƽ��ʱ![]() ����ȡ
����ȡ![]() ��
��![]() ����
����![]() ��һˮ�ϰ�����ƽ�ⳣ��
��һˮ�ϰ�����ƽ�ⳣ��![]() ��HF�ĵ��볣��
��HF�ĵ��볣��![]() ��
��![]() ����
����![]() ��Һ��笠�����ˮ��̶ȴ���Һ�����ԣ�
��Һ��笠�����ˮ��̶ȴ���Һ�����ԣ�
�ʴ�Ϊ��![]() �����ԣ�
�����ԣ�
![]() ��֪����ǰ��Һ��
��֪����ǰ��Һ��![]() ������þ�ʴﵽ
������þ�ʴﵽ![]() ʱ��
ʱ��![]() ��
��![]() ��
��![]() ��
��![]() ��
��
�ʴ�Ϊ��![]() ��
��
![]() ��NaOH��Һ����NaClO��
��NaOH��Һ����NaClO��![]() ��Ӧ�ɵ�
��Ӧ�ɵ�![]() ��ͬʱ���������ƺ��Ȼ��ƣ���Ӧ�Ļ�ѧ����ʽΪ��
��ͬʱ���������ƺ��Ȼ��ƣ���Ӧ�Ļ�ѧ����ʽΪ��![]() ��
��![]() ������������Ͻ�����������Ե��
������������Ͻ�����������Ե��![]() ��Һ
��Һ![]() ������ԭ��Ϊ��
������ԭ��Ϊ��![]()
![]()
![]() ��������
��������![]() ʧ��������
ʧ��������![]() ���缫��ӦΪ��
���缫��ӦΪ��![]() ��
��
�ʴ�Ϊ��![]() ��
��![]() ��
��

 ��ְٷְټ���ϵ�д�
��ְٷְټ���ϵ�д� �����ƻ���ĩ��̶�100��ϵ�д�
�����ƻ���ĩ��̶�100��ϵ�д� �ܿ���ȫ��100��ϵ�д�
�ܿ���ȫ��100��ϵ�д�����Ŀ����֪��![]() ʱ
ʱ
��ѧʽ |
|
|
|
����ƽ�ⳣ�� |
|
|
|
����˵����ȷ���� ( )
A. ����ϡ�����У�![]() ��С
��С
B. ![]() ��Һ�У�
��Һ�У�![]()
C. ������HCN��Һ�м���![]() ,������
,������![]()
D. ���ʵ���Ũ����ͬʱ![]()
����Ŀ��ijѧ����0.100 mol��L-1��NaOH����Һ�ζ�δ֪Ũ�ȵ����ᣬ������ɷֽ�Ϊ���¼�����
A����ȡ20.00 mL����������Һע��ྻ����ƿ��������2��3�η�̪��
B���ñ���Һ��ϴ�ζ���2��3�Σ�
C����ʢ�б���Һ�ļ�ʽ�ζ��̶ܹ��ã����ڵζ��ܼ���ʹ֮������Һ��
D��ȡ��NaOH��Һע���ʽ�ζ������̶ȡ�0������2��3 mL��
E������Һ������0����0�����¿̶ȣ����¶�����
F������ƿ���ڵζ��ܵ����棬�ñ�NaOH��Һ�ζ����յ㲢���µζ���Һ��Ŀ̶ȡ�
�ش��������⣺
��1����ȷ���������˳����(����ĸ�����д)_________��
��2����ȥ��ʽ�ζ��������ݵķ���Ӧ������ͼ�����е�________(����)��Ȼ�����ἷѹ������ʹ���첿�ֳ�����Һ��

��3���ζ�������,�۾�Ӧע��______________��
��4���жϵ���ζ��յ��ʵ��������__________________��
��5�����ݼ�¼���£�
�ζ����� | ������������/mL | ��NaOH��Һ��� | |
�ζ�ǰ�Ŀ̶�/mL | �ζ���Ŀ̶�/mL | ||
��һ�� | 20.00 | 0.40 | 20.50 |
�ڶ��� | 20.00 | 4.10 | 24.00 |
������ | 20.00 | 1.00 | 24.00 |
�����������ݣ��ɼ�����������Ũ��ԼΪ_____________(����С�������λ��)��
��6��������ʵ���У����в���(����������ȷ)����ɲⶨ���ƫ�͵���_____(����ĸ)��
A����ʽ�ζ���ʹ��ǰ��ˮϴ��δ�ô���������ϴ
B����ƿˮϴ��δ����
C����ʽ�ζ��ܼ��첿�������ݣ��ζ�����ʧ
D���ζ��յ����ʱ���Ӷ���
E���ζ��յ����ʱ���Ӷ���