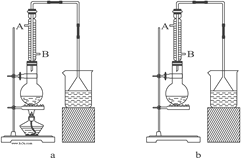
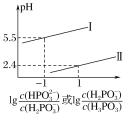
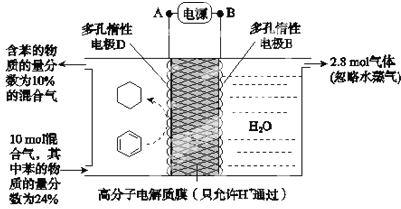
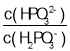��Ŀ����
����Ŀ��25 ��ʱ����Ũ�Ⱦ�Ϊ0.1 mol��L��1��NaOH��Һ������ֱ�ζ������Ϊ20 mLŨ�Ⱦ�Ϊ0.1 mol��L��1��HA��Һ��BOH��Һ���ζ���������Һ��pH��μ���Һ������仯��ϵ��ͼ��ʾ������˵������ȷ���ǣ�������
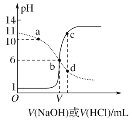
A.HAΪ���ᣬBOHΪǿ��
B.a��ʱ����Һ������Ũ�ȴ��ڹ�ϵ��c��BOH��<c��B����
C.b��ʱV��20
D.c��d������Һ��Ϻ���֮����ڹ�ϵ��c��H������c��OH������c��BOH��
���𰸡�D
��������
����ͼ�����������ͼ����HCl�ζ�BOH��Һ��ʵ��ͼ����NaOH�ζ�HA��Һ��HA��Һ��BOH��Һ��Ϊ0.1mol/L��������ʼ״̬�жϣ�HA��Һ��pHΪ1����HA��ȫ���룬��ǿ�ᣬBOH��ʼʱ��Һ��pHΪ11������ȫ���룬��BOH����������Һ�е��غ�˼�룬�ݴ��жϷ�����
����ͼ�����������ͼ����HCl�ζ�BOH��Һ��ʵ��ͼ����NaOH�ζ�HA��Һ��HA��Һ��BOH��Һ��Ϊ0.1mol/L��������ʼ״̬�жϣ�HA��Һ��pHΪ1����HA��ȫ���룬��ǿ�ᣬBOH��ʼʱ��Һ��pHΪ11������ȫ���룬��BOH�����
A��HA��ǿ�ᣬBOH�������A����
B��a������������0.1mol/L��HCl�ζ�20.00mL0.1mol/L��BOH��������Һ�еĵ���غ㣬c(H+)+c(B+)=c(OH)+c(Cl)����ҺΪ���ԣ���������BOH��c(BOH)> c(B+)����B����
C��b��ʱ������ͼ��ʱ��ҺpHֵΪ6����ҺΪ���ԣ�����ʱV=20������0.1mol/L��NaOH�ζ�20.00mL0.1mol/L��HA��Һ��˵����ʱΪ�ζ��յ㣬ǡ������NaA������HAΪǿ�ᣬ��ΪNaA������ˮ�⣬��ʱӦΪ���ԣ�����V=20�����ϣ���C����
D��c�����NaOH�ζ�HA��Һʱ����Һ��ʱΪ���ԣ����жϴ�ʱ��Һ�����Ϊ������NaOH��NaA��d�����HCl�ζ�BOH��Һʱ����Һ��ʱΪ���ԣ����ж���Һ�����ΪBCl������HCl������c��d��ʱ�μ�NaOH����HCl������ͬ������c��d��ʱ��ƽ��ʱNaOH��NaA��BCl��HCl�����ϱ���ͬ����Ϊ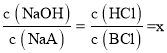 �����ݷ�Ӧ��ϵ�ͳ�ʼ������ϵ����֪c��d����Һ���֮������Ӧ��������Һ����ֹ�ϵΪx��c(NaCl)��1��c(NaA)��1��c(BCl)�����ݵ���غ㣬c(Na+)+c(H+)+c(B+)=c(OH)+c(A)+c(Cl)�����������غ㣬c(A)=c(B+)+c(BOH)��c(Na+)=c(Cl)���ۺϿ��ǣ�����c (H+)�Tc(OH)+c(BOH)����D��ȷ��
�����ݷ�Ӧ��ϵ�ͳ�ʼ������ϵ����֪c��d����Һ���֮������Ӧ��������Һ����ֹ�ϵΪx��c(NaCl)��1��c(NaA)��1��c(BCl)�����ݵ���غ㣬c(Na+)+c(H+)+c(B+)=c(OH)+c(A)+c(Cl)�����������غ㣬c(A)=c(B+)+c(BOH)��c(Na+)=c(Cl)���ۺϿ��ǣ�����c (H+)�Tc(OH)+c(BOH)����D��ȷ��
�ʴ�ѡ��D��

 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�