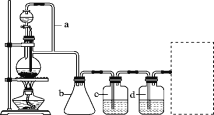
��Ŀ����
����Ŀ��C��CuO�ڸ����·�Ӧ��������Cu��![]() ��
��![]() ��CO���ֽ�
��CO���ֽ�![]() ̼�۸�
̼�۸�![]() ��ϣ���Ӳ���Թ��и����������¼��ȣ������ɵ�����ȫ��ͨ������NaOH��Һ�У����ռ���������壬�����Һ���ӵ�����Ϊ
��ϣ���Ӳ���Թ��и����������¼��ȣ������ɵ�����ȫ��ͨ������NaOH��Һ�У����ռ���������壬�����Һ���ӵ�����Ϊ![]() �����������ڱ�״���µ����Ϊ
�����������ڱ�״���µ����Ϊ![]() ������˵������ȷ����
������˵������ȷ����
A.��Ӳ���Թ��и����������¼��ȹ�������ʱ����![]() ̼�μ��˷�Ӧ
̼�μ��˷�Ӧ
B.�Թ��з���������������ԭ��Ӧ��ת�Ƶ���![]()
C.��Ӧ���Թ���ʣ��Ĺ��������������Ϊ![]()
D.��Ӧ����ͭ��������ͭ�������ʵ���Ϊ![]()
���𰸡�D
��������
���ɵ�����ȫ��ͨ������NaOH��Һ���ռ��������塣�����Һ���ص�![]() Ϊ������̼��������
Ϊ������̼��������![]() �����ʵ���Ϊ��
�����ʵ���Ϊ��![]() �����������ڱ�״����560mL����ΪCO���������CO�����ʵ���Ϊ��
�����������ڱ�״����560mL����ΪCO���������CO�����ʵ���Ϊ��![]() ��
��
A.����̼Ԫ���غ��֪���μӷ�Ӧ��Cԭ�ӵ����ʵ�������![]() ��CO�����ʵ���֮�ͣ����Բμӷ�Ӧ��̼Ԫ������Ϊ��
��CO�����ʵ���֮�ͣ����Բμӷ�Ӧ��̼Ԫ������Ϊ��![]() ����A��ȷ��
����A��ȷ��
B.��Ӧ��CԪ�ػ��ϼ����ߣ�ͭԪ�ػ��ϼ۽��ͣ�����ת�Ƶ������ʵ���Ϊ��![]() ����B��ȷ��
����B��ȷ��
C.���ɵ�![]() ��CO��������Ϊ��
��CO��������Ϊ��![]() �����Է�Ӧ���Թ��й�������������Ϊ��
�����Է�Ӧ���Թ��й�������������Ϊ��![]() ����C��ȷ��
����C��ȷ��
D.����ͭ�����ʵ���Ϊ��![]() ��������̼��һ����̼���е���ԭ�����ʵ���Ϊ��
��������̼��һ����̼���е���ԭ�����ʵ���Ϊ��![]() ����Ӧ����ԭ�Ӵ�����������ͭ�У�����������ͭ�����ʵ���Ϊ��
����Ӧ����ԭ�Ӵ�����������ͭ�У�����������ͭ�����ʵ���Ϊ��![]() ��ͭ�����ʵ���Ϊ��
��ͭ�����ʵ���Ϊ��![]() ����Ӧ����ͭ��������ͭ�������ʵ���Ϊ
����Ӧ����ͭ��������ͭ�������ʵ���Ϊ![]() ����D����
����D����
�ʴ�ΪD��

����Ŀ����������������ɴ�����Ⱦ����Ҫ���ʡ��о���������ķ�Ӧ����������������Ⱦ����Ҫ���塣
(1)NO�ڿ����д������·�Ӧ��2NO(g)+O2(g)=2NO2(g)��H��������Ӧ��������ɣ��䷴Ӧ��������ͼ��ʾ��
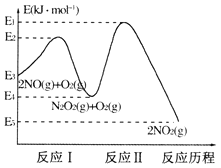
�ش��������⣺
��д����ӦI���Ȼ�ѧ����ʽ_________��
�ڷ�ӦI�ͷ�ӦII�У�һ���ǿ췴Ӧ������ٽ���ƽ��״̬������һ��������Ӧ������2NO(g)+O2(g)=2NO2(g)��Ӧ���ʵ��� _______��������ӦI��������ӦII�������Ը÷�Ӧ��ϵ�����¶ȣ������ܷ�Ӧ���ʷ�����������ԭ�������________����Ӧδʹ�ô�������
(2)�û���̿��ԭ����������������йط�ӦΪ��C(s)+2NO(g)![]() N2(g)+CO2(g)��������ܱ������м���һ�����Ļ���̿��NO��T��ʱ����������ʼŨ�ȼ�l0 min��20 min������ƽ��Ũ�������ʾ��
N2(g)+CO2(g)��������ܱ������м���һ�����Ļ���̿��NO��T��ʱ����������ʼŨ�ȼ�l0 min��20 min������ƽ��Ũ�������ʾ��
Ũ��/mol/L ʱ��/min | NO | N2 | CO2 |
0 | 0.100 | 0 | 0 |
10 | 0.040 | 0.030 | 0.030 |
20 | 0.032 | 0.034 | 0.017 |
��T��ʱ���÷�Ӧ��ƽ�ⳣ��Ϊ ________ (������λ��Ч����)��
����l0 minʱ����ֻ�ı�ijһ����ʹƽ�ⷢ���ƶ���20 minʱ���´ﵽƽ�⣬��ı��������________
����20 minʱ�������¶Ⱥ�������������ٳ���NO��N2��ʹ���ߵ�Ũ�Ⱦ�������ԭ������������ʱ��Ӧv��____v��������>������<������=������
(3)NO2��������ƽ�⣺2NO2(g)![]() N2O4(g) ��H<0����һ��������NO2��N2O4��������������Եķ�ѹ(��ѹ=��ѹ�����ʵ�������)�����¹�ϵ��v(NO2)=k1��p2(NO2)��v(N2O4)=k2��p(N2O4)����Ӧ�����������ѹ��ϵ��ͼ��ʾ��
N2O4(g) ��H<0����һ��������NO2��N2O4��������������Եķ�ѹ(��ѹ=��ѹ�����ʵ�������)�����¹�ϵ��v(NO2)=k1��p2(NO2)��v(N2O4)=k2��p(N2O4)����Ӧ�����������ѹ��ϵ��ͼ��ʾ��
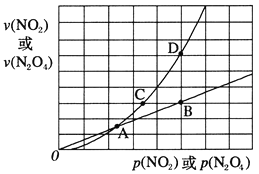
һ���¶��£�k1��k2��ƽ�ⳣ��Kp(ѹ��ƽ�ⳣ������ƽ���ѹ����ƽ��Ũ�ȼ���)��Ĺ�ϵ��k1=____������ͼ������У�ָ���ܱ�ʾ��Ӧ�ﵽƽ��״̬�ĵ��� ______��
����Ŀ�����ݻ�Ϊ1 L�ĺ����ܱ������г���CO(g)��H2O(g)��������Ӧ��CO(g) + H2O(g)![]() CO2(g) + H2(g) ��H<0������ʵ���������±���
CO2(g) + H2(g) ��H<0������ʵ���������±���
ʵ�� ��� | �¶�/�� | ��ʼʱ���ʵ���/mol | ƽ��ʱ���ʵ���/mol | |
n(CO) | n(H2O) | n(CO2) | ||
�� | 700 | 0.40 | 0.10 | 0.090 |
�� | 800 | 0.10 | 0.40 | 0.080 |
�� | 800 | 0.20 | 0.30 | a |
�� | 900 | 0.10 | 0.15 | b |
����˵����ȷ����
A. ʵ����У���5 minʱ���n (CO2) =0.050 mol����0��5 minʱ���ڣ���H2��ʾ��ƽ����Ӧ������ (H2) = 5.0��10��2 mol/(L��min)
B. ʵ����У��������·�Ӧ��ƽ�ⳣ��K =2.0
C. ʵ����У��ﵽƽ��ʱ��CO��ת����Ϊ60%
D. ʵ����У��ﵽƽ��ʱ��b��0.060