��Ŀ����
��14�֣�ijͬѧ��ͨ��������ͼ��ʾװ�ã��г�װ����ȥ��ʵ�飬̽��SO2��Na2O2��Ӧ�IJ���

���������װ��B������ װ��D������
����μ��鷴Ӧ���Ƿ���O2����
��C�й������������¼��裺����1��ֻ��Na2SO3 ����2��ֻ��Na2SO4
����3��
��1����������2���У���Ӧ����ʽΪ
��2����Na2O2��Ӧ��ȫ��Ϊȷ��C�й������ijɷ֣���ͬѧ�������ʵ�飺
�ó����ۣ�����2����
�÷����Ƿ���� ����ǡ�����������
��3��������2��������Ӧǰ��C������6.4g �������10g�������ʵ�Na2O2�����У�Na2O2����������Ϊ �����������ʲ���SO2������Ӧ��

���������װ��B������ װ��D������
����μ��鷴Ӧ���Ƿ���O2����
��C�й������������¼��裺����1��ֻ��Na2SO3 ����2��ֻ��Na2SO4
����3��
��1����������2���У���Ӧ����ʽΪ
��2����Na2O2��Ӧ��ȫ��Ϊȷ��C�й������ijɷ֣���ͬѧ�������ʵ�飺
�ó����ۣ�����2����

�÷����Ƿ���� ����ǡ�����������
��3��������2��������Ӧǰ��C������6.4g �������10g�������ʵ�Na2O2�����У�Na2O2����������Ϊ �����������ʲ���SO2������Ӧ��

(1) Na2O2 + SO2 ="==" Na2SO4

��Ũ��������ˮ�ԣ������ն��������е�ˮ�֣���ֹˮ������������Ʒ�Ӧ�����Ŷ�������ʯ���Ǹ������������ˮ�֣���ֹˮ�����ĸ��ţ���ʯ���Ǽ������ʣ������ն���������������壬��ֹ��Ⱦ�������ʴ�Ϊ������SO2���壬��ֹˮ������Na2O2��Ӧ����ֹ�����е�ˮ�����Ͷ�����̼����Cװ����Na2O2��Ӧ��ͬʱ���չ�����SO2��������Ⱦ������
���ô����ǵ�ľ���ɼ����������ʴ�Ϊ���ô����ǵ�ľ����������ܿ�a���۲����Ƿ�ȼ�գ�
�������������Ƴ�������3���Ǻ����������ƺ������ƵĻ����ʴ�Ϊ����Na2SO3��Na2SO4��
��1���������ƺͶ�������Ӧ���������Ƶ�ԭ������ʽΪ��Na2O2+SO2=Na2SO4���ʴ�Ϊ��Na2O2+SO2=Na2SO4��
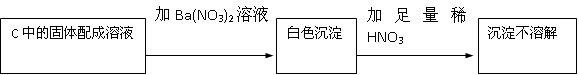
��2�����ɵİ�ɫ������������������ᱵ����������Ὣ֮����Ϊ���ᱵ�����ܵó����ۣ�������Na2SO4���ʴ�Ϊ����HNO3�������ԣ��ݴ˲���ȷ��������Na2SO3����Na2SO4������У�
��3��������ݣ�m2-m1�����ڲμӷ�Ӧ��SO2�������ƶ�����Na2SO3������Ϊ��m2-m1����126/64g�����Ǹ÷�Ӧ������SO2ͬʱ��O2���������ԣ� m 2-m 1�������ڲμӷ�Ӧ��SO2�������ʸ����㲻�������ʴ�Ϊ����Ϊ�÷�Ӧ������SO2ͬʱ��O2���������ԣ�m2-m1�������ڲμӷ�Ӧ��SO2�������ʸ����㲻������
��4��������2�������������ƺͶ�������ķ�ӦΪ��Na2O2+SO2=Na2SO4�������ص��ڲμӷ�Ӧ��SO2��������μӷ�Ӧ��Na2O2���ʵ���n��Na2O2��=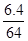 =0.1mol������Ϊ7.8g����������Ϊ78����
=0.1mol������Ϊ7.8g����������Ϊ78����
���������⿼���˳����������ȡ��ʵ��װ�õ�ѡ����һ���ۺ�֪ʶ��Ŀ���ѶȽϴ�
���ô����ǵ�ľ���ɼ����������ʴ�Ϊ���ô����ǵ�ľ����������ܿ�a���۲����Ƿ�ȼ�գ�
�������������Ƴ�������3���Ǻ����������ƺ������ƵĻ����ʴ�Ϊ����Na2SO3��Na2SO4��
��1���������ƺͶ�������Ӧ���������Ƶ�ԭ������ʽΪ��Na2O2+SO2=Na2SO4���ʴ�Ϊ��Na2O2+SO2=Na2SO4��
��2�����ɵİ�ɫ������������������ᱵ����������Ὣ֮����Ϊ���ᱵ�����ܵó����ۣ�������Na2SO4���ʴ�Ϊ����HNO3�������ԣ��ݴ˲���ȷ��������Na2SO3����Na2SO4������У�
��3��������ݣ�m2-m1�����ڲμӷ�Ӧ��SO2�������ƶ�����Na2SO3������Ϊ��m2-m1����126/64g�����Ǹ÷�Ӧ������SO2ͬʱ��O2���������ԣ� m 2-m 1�������ڲμӷ�Ӧ��SO2�������ʸ����㲻�������ʴ�Ϊ����Ϊ�÷�Ӧ������SO2ͬʱ��O2���������ԣ�m2-m1�������ڲμӷ�Ӧ��SO2�������ʸ����㲻������
��4��������2�������������ƺͶ�������ķ�ӦΪ��Na2O2+SO2=Na2SO4�������ص��ڲμӷ�Ӧ��SO2��������μӷ�Ӧ��Na2O2���ʵ���n��Na2O2��=
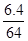 =0.1mol������Ϊ7.8g����������Ϊ78����
=0.1mol������Ϊ7.8g����������Ϊ78�������������⿼���˳����������ȡ��ʵ��װ�õ�ѡ����һ���ۺ�֪ʶ��Ŀ���ѶȽϴ�

��ϰ��ϵ�д�
�����Ŀ
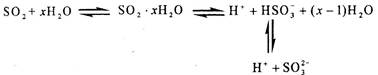

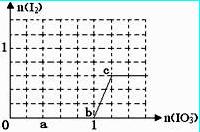
 HSO3����Һ����μ���KIO3��Һ�������KIO3��������I2�����ʵ����Ĺ�ϵ��������ͼ��ʾ��
HSO3����Һ����μ���KIO3��Һ�������KIO3��������I2�����ʵ����Ĺ�ϵ��������ͼ��ʾ��