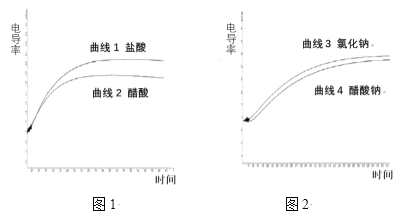
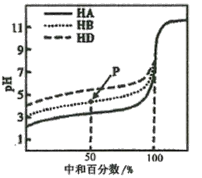
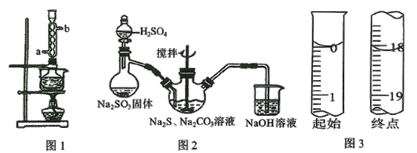
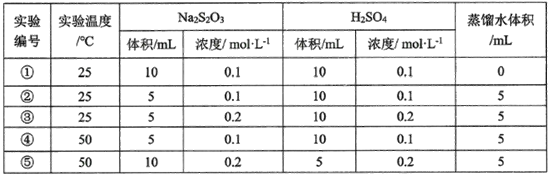
��Ŀ����
����Ŀ�������£���474.8gˮ���ܽ�25.2g���ᾧ��(H2C2O4��2H2O����Է�������Ϊ126)���õ�һ�ݲ���Ũ��Һ������Һ�ܶ�Ϊ2.0g/cm3��
��1�����Ϲ��̵õ��IJ���Ũ��Һ�����ʵ���Ũ��Ϊ___mol/L��
��2������������Ũ��Һ����0.1mol/L�IJ�����Һ450mL��
��ʵ�����õ��IJ��������н�ͷ�ιܡ��ձ�����Ͳ��___��
��Ӧ��ȡŨ��Һ�����Ϊ___mL��
����������У��ᵼ�����Ƶ�0.1mol/L�IJ�����ҺŨ��ƫ�ߵ���___������ĸ����
A������Ũ��Һ��ʹ�õIJ��ᾧ��ʧȥ���ֽᾧˮ
B��ʹ���������������ķ����������ᾧ��
C������ƿδ�������ʹ��
D����ȡŨ��Һʱ���ӿ̶���
��3��H2C2O4��2H2O�����ֽ⣬�ֽ�����Ϊ���������һ�ֲ�����ʹ����ʯ��ˮ����ǣ���д��H2C2O4��2H2O���ȷֽ�Ļ�ѧ����ʽ___��
��4������Һ����ȡ10.00mL����K2Cr2O7��Һ��ǡ����20.00mL0.1mol/L�IJ�����Һ��Ӧ����ԭ����ΪCr3+����������ΪCO2���ڴ�ʵ���У���������___�ԣ�������K2Cr2O7��Һ�����ʵ���Ũ��Ϊ___mol/L������С�����2λ����
���𰸡�0.8 500mL����ƿ�������� 62.5 AD H2C2O4��2H2O![]() 3H2O��+CO��+CO2�� ��ԭ 0.07
3H2O��+CO��+CO2�� ��ԭ 0.07
��������
��1������n=![]() ����������ʵ���������c=
����������ʵ���������c=![]() ����ɵã�
����ɵã�
��2����ʵ����û��450mL����ƿ������450mL 0.1mol/L����KMnO4��ҺӦѡ��500mL����ƿ�����Ʋ����м��㡢�������ܽ⡢��ȴ����Һ��ϴ�ӡ����ݡ�ҡ�ȵȲ�����
����ϡ�Ͷ��ɿ�֪�����ƹ����в�������ʵ������䣻
������c=![]() ͨ���������ʵ����ʵ�������Һ����仯������
ͨ���������ʵ����ʵ�������Һ����仯������
��3������������ԭ��Ӧ���ɣ�������ԭ��Ӧ����Ԫ�ػ��ϼ����ߣ���Ȼ��Ԫ�ػ��ϼ۽��ͷ����жϣ�
��4���ɵ�ʧ������Ŀ�غ���ɼ���ɵá�
��1��25.2g���ᾧ���в�������ʵ���Ϊ![]() =0.2mol�������£���474.8gˮ���ܽ�25.2g���ᾧ��õ�����Ũ��Һ������Ϊ500g�������Ũ��Һ�����ʵ���Ũ��Ϊ
=0.2mol�������£���474.8gˮ���ܽ�25.2g���ᾧ��õ�����Ũ��Һ������Ϊ500g�������Ũ��Һ�����ʵ���Ũ��Ϊ![]() =0.8mol/L���ʴ�Ϊ��0.8��
=0.8mol/L���ʴ�Ϊ��0.8��
��2����ʵ����û��450mL����ƿ������450mL 0.1mol/L����KMnO4��ҺӦѡ��500mL����ƿ�����Ʋ����м��㡢�������ܽ⡢��ȴ����Һ��ϴ�ӡ����ݡ�ҡ�ȵȲ�����һ����������ƽ��������ҩ��ȡ��ҩƷ�����ձ����ܽ⣨������Ͳ��ȡˮ������ȴ��ת�Ƶ�500mL����ƿ�У����ò���������������ˮ��Һ�����̶���1��2cmʱ�����ý�ͷ�ιܵμӣ�������Ҫ������Ϊ��������ƽ��ҩ�ס��ձ�����������500mL����ƿ����ͷ�ιܣ�������֪��Ҫʹ�õ�������500mL����ƿ����ͷ�ιܣ��ʴ�Ϊ��500mL����ƿ����ͷ�ιܣ�
����ϡ�Ͷ��ɿ�֪�����ƹ����в�������ʵ������䣬��Ũ��Һ�����ΪVml��ɵ����¹�ϵʽ��0.8mol/L��V��10��3L=0.1mol/L��500��10��3L�����V=62.5���ʴ�Ϊ��62.5��
��A������Ũ��Һ��ʹ�õIJ��ᾧ��ʧȥ���ֽᾧˮ�ᵼ�²������ʵ���ƫ��������ҺŨ��ƫ�ߣ�����ȷ��
B��ʹ���������������ķ����������ᾧ��ᵼ�����Ʋ��������ƫС�����ʵ���ƫС��������ҺŨ��ƫС���ʴ���
C����������Һ��������Ҫ��������ˮ������ϡ�Ͷ��ɿ�֪����ƿδ�������ʹ�ò�������ʵ������䣬��������ҺŨ����Ӱ�죬�ʴ���
D����ȡŨ��Һʱ���ӿ̶��ߣ��ᵼ����ȡŨ��Һ���ƫ�������ʵ���ƫ��������ҺŨ��ƫ�ߣ�����ȷ��
AD��ȷ���ʴ�Ϊ��AD��
��3��H2C2O4��̼Ԫ�صĻ��ϼ�Ϊ+�ۣ���H2C2O4��2H2O�����ֽ⣬�ֽ�����Ϊ���������һ�ֲ�����ʹ����ʯ��ˮ����ǿ�֪����Ӧ���ж�����̼���ɣ�̼Ԫ�ػ��ϼ����ߣ���������ԭ��Ӧ���ɿ�֪��Ӧ�����ɶ�����̼��ˮ�����������⣬��Ӧ��������һ����̼���ɣ���Ӧ�Ļ�ѧ����ʽΪH2C2O4��2H2O![]() 3H2O��+CO��+CO2�����ʴ�Ϊ��H2C2O4��2H2O
3H2O��+CO��+CO2�����ʴ�Ϊ��H2C2O4��2H2O![]() 3H2O��+CO��+CO2����
3H2O��+CO��+CO2����
��4���ɻ�ԭ����ΪCr3+����������ΪCO2��֪����Ӧ��K2Cr2O7Ϊ������������Ϊ��ԭ����������K2Cr2O7��Һ��Ũ��Ϊc���ɵ�ʧ������Ŀ�غ�ɵã�0.002L��0.1mol/L��2����4��3��=0.001L��cmol/L��2����6��3�������c��0.07mol/L���ʴ�Ϊ����ԭ��0.07��