��Ŀ����
25��ʱ������ĵ���ƽ�ⳣ�����±���
�����й�˵����ȷ����(����)
A�������ʵ���Ũ�ȵĸ���Һ��pH��ϵΪ�� pH(NaCN)��pH(Na2CO3)��pH(CH3COONa)
B��a mol/L HCN��a mol/L NaOH��Һ�������Ϻ�������Һ������
C��������������μ�ˮϡ�ͣ���Һ��������ʼ�ձ��ּ�С
D����Na2CO3��Һ�У�c(OH-)�� c(H+)�� c(HCO3-) �� 2c(H2CO3)
| ���� | CH3COOH | HCN | H2CO3 |
| Ka | 1.8��10��5 | 4.9��10��10 | K1��4.3��10��7 K2��5.6��10��11 |
�����й�˵����ȷ����(����)
A�������ʵ���Ũ�ȵĸ���Һ��pH��ϵΪ�� pH(NaCN)��pH(Na2CO3)��pH(CH3COONa)
B��a mol/L HCN��a mol/L NaOH��Һ�������Ϻ�������Һ������
C��������������μ�ˮϡ�ͣ���Һ��������ʼ�ձ��ּ�С
D����Na2CO3��Һ�У�c(OH-)�� c(H+)�� c(HCO3-) �� 2c(H2CO3)
D
���������A�����ݵ���ƽ�ⳣ����֪����ǿ��˳��Ϊ��CH3COOH��HCN��HCO3-������Խ������Ӧ�������������ˮ��̶�Խ����Һ��PHԽ�����ʵ���Ũ�ȵĸ���ҺpH��ϵΪ��pH��Na2CO3����pH��NaCN����pH��CH3COONa������A����B������Ϻ���ΪNaCN����ǿ�������Σ����Լ��ԡ���B����C������������������ʣ���ˮ�ٽ����룬����������μ�ˮ����Һ�ĵ�������������С��pH�ȼ�С��������ĵ���̶�������C����D����̼������Һ�У�̼���ˮ�⣬�γ�HCO3-��H2CO3�����������غ��֪��c(OH-)�� c(HCO3-) �� 2c(H2CO3)+c(H+)����D��ȷ��

��ϰ��ϵ�д�
�����Ŀ
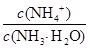 ��ֵ �����������С�����䡱����
��ֵ �����������С�����䡱����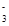 ��SiO32-
��SiO32-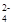 ��CO
��CO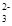
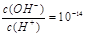 ����Һ�д���Al3+��NH4+��Cl-��NO3-
����Һ�д���Al3+��NH4+��Cl-��NO3- ���ʱ�
���ʱ�