��Ŀ����
ijʵ��С�������50 mL 1.0 mol/L�����50 mL 1.1 mol/L ����������Һ����ͼװ���н����кͷ�Ӧ���ڴ��ձ��ײ�������ĭ����(��ֽ��)��ʹ�����С�ձ���������ձ�������ƽ��Ȼ�����ڴ�С�ձ�֮����������ĭ����(��ֽ��)�����ձ�������ĭ���ϰ�(��Ӳֽ��)���ǰ壬�ڰ��м俪����С�ף�����ʹ�¶ȼƺͻ��β��������ͨ����ͨ���ⶨ��Ӧ���������ų��������ɼ����к��ȡ��Իش��������⣺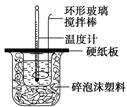
��1����ʵ�������Թ�����NaOH��ԭ��̲���˵��Ϊ��֤������ȫ���к͡����ʣ������ڷ�Ӧ������Ϊ�з�������������������ڷ�Ӧ�лӷ������õ��к��� (�ƫ����ƫС�����䡱)��
��2�����к��Ȳⶨʵ���д�����ˮϴ���¶ȼ��ϵ�����IJ��裬���˲������裬���õ��к��Ȼ� (�ƫ����ƫС�����䡱)��
��3�����õ�Ũ�ȵĴ�����NaOH��Һ��Ӧ�����õ��к��Ȼ� (�ƫ����ƫС�����䡱)����ԭ���� ��
��4����ʵ��С����������ʵ�飬ÿ��ȡ��Һ��50 mL������¼��ԭʼ����(���±�)��
| ʵ����� | ��ʼ�¶�t1/�� | ��ֹ�¶�(t2)/�� | �²�(t2��t1)/�� | ||
| ���� | NaOH��Һ | ƽ��ֵ | |||
| 1 | 25.1 | 24.9 | 25.0 | 31.6 | 6.6 |
| 2 | 25.1 | 25.1 | 25.1 | 31.8 | 6.7 |
| 3 | 25.1 | 25.1 | 25.1 | 31.9 | 6.8 |
��֪���ᡢNaOH��Һ�ܶȽ���Ϊ1.00g/cm3���кͺ���Һ�ı�����c��4.18��10��3kJ/(g����)����÷�Ӧ���к���Ϊ��H�� �����ݼ�������д�����кͷ�Ӧ���Ȼ�ѧ����ʽ ��
(1)ƫС (2) ƫС��3��ƫС��4�� ��56.01 kJ/mol
���������������1������Ϊ�з������������������ڷ�Ӧ�лӷ���������HCl�������������ɵ�ˮ�����ʵ���ƫС���ʲ�õ��к��Ȼ�ƫС��
��2��û����ˮϴ���¶ȼ��ϵ�������Һ��������������ʵ���ƫС���ų�������ƫС����õ��к�����ֵƫС��
��3�����ڴ���Ϊ���ᣬ�������Ҫ������������ɲ�õ��к���ƫС��
�ʴ�Ϊ��ƫС���ô���������ᣬ�������Ҫ������������ɲ�õ��к���ƫС��
��4���������β������ݶ�����Ч�ģ������²��ƽ��ֵΪ��
��H����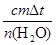 ����
����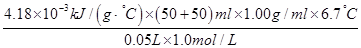 ����56.01 kJ/mol��
����56.01 kJ/mol��
�����к��ȵĸ����֪���Ȼ�ѧ����ʽΪ��HCl��aq��+NaOH��aq��=NaCl��aq��+H2O��l����H=-57.3KJ/mol��
�ʴ�Ϊ��-56.01 kJ/mol��HCl��aq��+NaOH��aq��=NaCl��aq��+H2O��l����H=-57.3KJ/mol��
���㣺���⿼�����к��ȵij���

 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д��״���һ�ֳ��õ�ȼ�ϣ���ҵ�Ͽ�����CO��H2��һ�������ºϳɼ״���
��1����֪CO��g����H2��g����CH3OH��1����ȼ���ȡ�H�ֱ�Ϊ��-283.0kJ��mol��-285.8 kJ/mol��-726.5kJ/mol����CO�ϳɼ״����Ȼ�ѧ����ʽΪ�� ��
��2���ں����ܱ�������CO��H2������Ӧ���ɼ״���������Ũ���ڲ�ͬ�����µı仯״����ͼ��ʾ����ʼʱ������Ũ�����ߺ�8���Ӻ�״���Ũ������δ������4���Ӻ�8���Ӹı��������ͬ���� 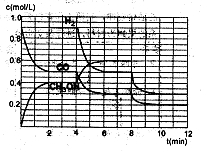
������˵����ȷ����
| A����ʼʱn��H2��Ϊ1��7mol |
| B����������ѹǿ�㶨ʱ��˵����Ӧ�ﵽƽ��״̬ |
| C��4����ʱ���ı�������������¶� |
| D��7����ʱ��v��CO��=v��CH3OH�� |
����3minʱ�÷�Ӧ��ƽ�ⳣ��K= ����������
����ͼ�л���8~12min֮��c��CH3OH������
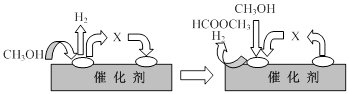
��2��2009�꣬�й��ڼ״�ȼ�ϵ�ؼ����ϻ��ͻ�ƣ���װ���Ժ�����ؼ�����ʽ��ѣ���װ��ԭ����ͼ�ס�
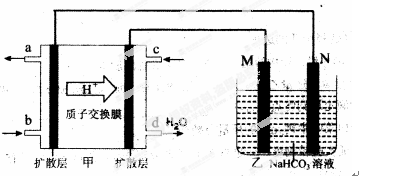
�ٸõ�صĸ�����ӦʽΪ�� ��
���ҳ���һ����Ʒ���桰�ۻ���װ�ã������ֱ�Ϊ����Ʒ��ʯī��
M�缫�IJ����� ��������Ʒ���桰�ۻ���ʱ�ķ�ӦʽΪ�� ��
�Ҵ������DZ��㷺ʹ�õ��������ȼ�ϣ���ҵ�����Ҵ���һ�ַ�Ӧԭ��Ϊ��
2CO(g) + 4H2(g) CH3CH2OH(g) + H2O(g) ��H =" ��256.1" kJ��mol��1
CH3CH2OH(g) + H2O(g) ��H =" ��256.1" kJ��mol��1
��֪��CO(g) + H2O(g) CO2(g)+H2(g) ��H=" ��41.2" kJ��mol��1
CO2(g)+H2(g) ��H=" ��41.2" kJ��mol��1
��1����CO2(g)��H2(g)Ϊԭ��Ҳ�ɺϳ��Ҵ������Ȼ�ѧ����ʽ���£�
2CO2(g) +6H2(g) CH3CH2OH(g) +3H2O(g) ��H = ��
CH3CH2OH(g) +3H2O(g) ��H = ��
��2������ʹ���Ҵ����Ͳ����ܼ���NOx���ŷţ���ʹNOx����Ч������Ϊ�����������Ҫ���⡣
��ij�о�С����ʵ������Ag�C ZSM�C 5Ϊ���������NOת��ΪN2��ת�������¶ȱ仯�������ͼ������ʹ��CO���¶ȳ���800�棬����NO��ת���ʽ��ͣ�����ܵ�ԭ��Ϊ ����n(NO)/n(C O)=1�������£�Ӧ���Ƶ�����¶��� ���ҡ�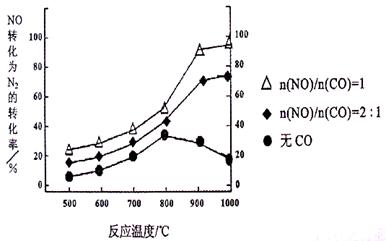
���û���̿��ԭ��������������йط�ӦΪ��C (s) +2NO2(g) N2 (g) + CO2 (g)��ij�о�С����ij�ܱ������м��������Ļ���̿��NO������( T1��)�����·�Ӧ����Ӧ���е���ͬʱ���ø����ʵ�Ũ�����£�
N2 (g) + CO2 (g)��ij�о�С����ij�ܱ������м��������Ļ���̿��NO������( T1��)�����·�Ӧ����Ӧ���е���ͬʱ���ø����ʵ�Ũ�����£�
| �� Ũ��/mol?L��1 ʱ��/min | NO | N2 | CO2 |
| 0 | 1.00 | 0 | 0 |
| 20 | 0.40 | 0.30 | 0.30 |
| 30 | 0.40 | 0.30 | 0.30 |
| 40 | 0.32 | 0.34 | 0.17 |
| 50 | 0.32 | 0.34 | 0.17 |
I�����ݱ������ݣ���Ӧ��ʼ��20min��v(NO)��ʾ�ķ�Ӧ����Ϊ (������λ��Ч����)��T1��ʱ�÷�Ӧ��ƽ�ⳣ��Ϊ (������λ��Ч����)��
II��30min�ı�ijһ��������Ӧ���´ﵽƽ�⣬��ı������������ ����ͼ��ʾCO2���淴Ӧ����[v��(CO2)]�淴Ӧʱ��ı仯��ϵͼ������ͼ�л�����30min�ı���������ʱ����40minʱ���ٴδﵽƽ��ı仯���ߡ�

����������£��磩 ��ã��磩�Ŀ��淴Ӧ�У�ͼ���ں���������ƽ��ʱ��B������������£�����ѹǿ��p���Ĺ�ϵ��ͼ�ұ�ʾ��һ�������´ﵽƽ�⣨���������������1ʱ�̸ı�Ӱ��ƽ�����һ���������½�����ƽ��ķ�Ӧ���̣��й�������ȷ����
��ã��磩�Ŀ��淴Ӧ�У�ͼ���ں���������ƽ��ʱ��B������������£�����ѹǿ��p���Ĺ�ϵ��ͼ�ұ�ʾ��һ�������´ﵽƽ�⣨���������������1ʱ�̸ı�Ӱ��ƽ�����һ���������½�����ƽ��ķ�Ӧ���̣��й�������ȷ����
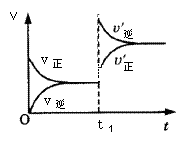
�� ��
| A��n<q | B��n>q |
| C������ӦΪ���ȷ�Ӧ | D��X���Y�㷴Ӧ���ʿ� |
һ�������£������Ϊ10L���ܱ������У�1 molX��1 molY������Ӧ��2x��g��+Y��g�� Z��g������60s�ﵽƽ�⣬����0��3mol Z������˵����ȷ����
Z��g������60s�ﵽƽ�⣬����0��3mol Z������˵����ȷ����
| A����Ӧ����30 sʱ������Ӧ���ʵ����淴Ӧ���� |
| B����Ӧ����80 sʱ���淴Ӧ���ʴ�������Ӧ���� |
| C����Ӧ����60 sʱ��X�����ʵ���Ũ��Ϊ0��04 mol/L |
| D����Ӧ����60 sʱ��Y��ת����Ϊ70�� |
 W (s) + 2H2O (g)����H�� +66.0 kJ�� mol��1
W (s) + 2H2O (g)����H�� +66.0 kJ�� mol��1 2NH3(g) ��H<0��ijʵ�齫3.0 mol N2(g)��4. 0 mol H2(g)�����ݻ�Ϊ10L���ܱ������У����¶�T1�·�Ӧ�����H2�����ʵ����淴Ӧʱ��ı仯����ͼ��ʾ��
2NH3(g) ��H<0��ijʵ�齫3.0 mol N2(g)��4. 0 mol H2(g)�����ݻ�Ϊ10L���ܱ������У����¶�T1�·�Ӧ�����H2�����ʵ����淴Ӧʱ��ı仯����ͼ��ʾ��
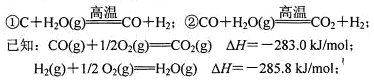
 HCOOCH3(g)+2H2(g) ��H>0
HCOOCH3(g)+2H2(g) ��H>0