��Ŀ����
(14 ��) һ����̼���㷺Ӧ����ұ��ҵ�͵��ӹ�ҵ��
�Ÿ�¯��������Ϊ�ձ��������������ط�Ӧ���Ȼ�ѧ����ʽ���£�
4CO(g)��Fe3O4(s)��4CO2(g)��3Fe(s) ��H="a" kJ��mol��1
CO(g)��3Fe2O3(s)��CO2(g)��2Fe3O4(s) ��H="b" kJ��mol��1
��Ӧ3CO(g)��Fe2O3(s)��3CO2(g)��2Fe(s)�ġ�H= kJ��mol��1(�ú�a��b �Ĵ���ʽ��ʾ)��
�Ƶ��ӹ�ҵ��ʹ�õ�һ����̼���Լ״�Ϊԭ��ͨ�����⡢�ֽ�������Ӧ�õ���
��һ����2CH3OH(g) HCOOCH3(g)+2H2(g) ��H>0
HCOOCH3(g)+2H2(g) ��H>0
�ڶ�����HCOOCH3(g) CH3OH(g) +CO(g) ��H>0
CH3OH(g) +CO(g) ��H>0
�ٵ�һ����Ӧ�Ļ�����������ͼ��ʾ��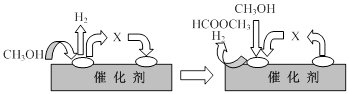
ͼ���м����X�Ľṹ��ʽΪ ��
���ڹ�ҵ�����У�Ϊ���CO�IJ��ʣ��ɲ�ȡ�ĺ�����ʩ�� ��
��Ϊ��������о�����CO��ԭ��������ʯ����Ӧ��������ʵ�X������������ͼ��ͼ��ʾ��X����������������ж�ij��̬�����Ƿ���ڣ���ͬ��̬���ʳ�������������Dz�ͬ������Ӧ�������е�һ�ֲ����������ᷴӦ���������Σ��÷�Ӧ�����ӷ���ʽΪ ��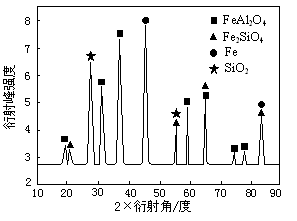
��ij������Ʒ����Ni2O340%������ΪSiO2��ͨ����ԭ���ᴿ������������ʣ�������CO��33.2 g��Ʒ�ڼ��������»�ԭΪ������Ȼ���ڳ�����ʹ�����е�Ni��CO��ϳ�Ni(CO)4���е�43 �棩������180 ��ʱʹNi(CO)4���·ֽ���������ʡ�
��������������CO�����ʵ���֮��Ϊ ��
��Ϊ��ȫ�������ҵ��������Կ����е�CO���м�⡣
�ٷۺ�ɫ��PdCl2��Һ���Լ��������������CO���������к�CO������Һ�л������ɫ��Pd������ÿ����5.3gPd��������Ӧת�Ƶ�����Ϊ ��
��ʹ�õ绯ѧһ����̼���崫����������������CO��������ṹ��ͼ��ʾ�����ִ���������ԭ���ԭ������õ�صĸ�����ӦʽΪ ��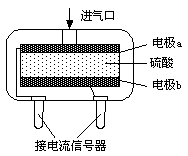
��(2a+b)/3
�Ƣ�HCHO
�������¶ȣ�����ѹǿ
��FeAl2O4+8H+��Fe2++2Al3++4H2O
��3:8
�ɢ�0.1mol����0.1NA��
��CO+H2O��2e����CO2+2H+
��ÿ��2�֣���14�֣�
���������������1������ʽ���١�2+�ڣ���3�õ�3CO(g)��Fe2O3(s)��3CO2(g)��2Fe(s)����HҲ�ǣ���2���ӵ�һ��ͼ���Եó��״�ʧȥ�����õ�X��Ӧ�����ɼ�ȩHCHO���ӵڶ�����Ӧ�������¶ȡ�����ѹǿƽ�������ƶ���ת������ߣ���3����������е�һ�ֲ����������ᷴӦ���������Ρ�ȷ����Ӧ���ﺬ��FeAl2O4�������ᷴӦ����Ϊ�Ȼ��������Ȼ�������4��������ӦʽΪ3CO��Ni2O3��3CO2��2 Ni��Ni + 4CO =Ni(CO)4������1mol Ni����3/2molCO���ڶ�������4molCO����������������CO�����ʵ���֮��Ϊ3:8����5��ת�Ƶĵ�����Ϊ5.3��106��2=0.1mol����صĸ���ΪCOʧȥ����ת��ΪCO2��
���㣺���黯ѧԭ���ۺ��й����⡣

����Ͱ��ڹ�����ռ����Ҫ��λ��
��1���Ʊ��ϳɰ�ԭ����H2�����ü�������ת��������Ҫת����Ӧ���£�
CH4(g) + H2O(g)  CO(g) + 3H2(g) ��H =" +206.2" kJ/mol
CO(g) + 3H2(g) ��H =" +206.2" kJ/mol
CH4(g) + 2H2O(g)  CO2(g) +4H2(g) ��H = +165.0kJ/mol
CO2(g) +4H2(g) ��H = +165.0kJ/mol
������Ӧ����ԭ�����е�CO��ʹ���ϳɴ����ж��������ȥ����ҵ�ϳ����ô���������CO��ˮ������Ӧ�����׳�ȥ��CO2��ͬʱ�ֿ��Ƶõ�����������ķ������˷�Ӧ��Ϊһ����̼�任��Ӧ���÷�Ӧ���Ȼ�ѧ����ʽ�� ��
��2����ҵ�������ص�ԭ������NH3��CO2Ϊԭ�Ϻϳ�����[CO(NH2)2]����Ӧ�Ļ�ѧ����ʽΪ��2NH3 (g)+ CO2 (g)  CO(NH2)2 (l) + H2O (l)���÷�Ӧ��ƽ�ⳣ�����¶ȹ�ϵ���£�
CO(NH2)2 (l) + H2O (l)���÷�Ӧ��ƽ�ⳣ�����¶ȹ�ϵ���£�
| T / �� | 165 | 175 | 185 | 195 |
| K | 111.9 | 74.1 | 50.6 | 34.8 |
�� ��Ӧ�Ȧ�H���������������������_______0��
�� ��һ���¶Ⱥ�ѹǿ�£���ԭ�����е�NH3��CO2�����ʵ���֮�ȣ���̼�ȣ�
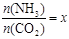 ����ͼ�ǰ�̼�ȣ�x����CO2ƽ��ת���ʣ������Ĺ�ϵ����ͼ�е�B�㴦��NH3��ƽ��ת���ʡ�
����ͼ�ǰ�̼�ȣ�x����CO2ƽ��ת���ʣ������Ĺ�ϵ����ͼ�е�B�㴦��NH3��ƽ��ת���ʡ�
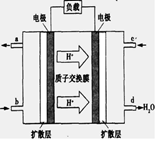
��3����֪����ȼ�ϵ�صĹ���ԭ������ͼ��ʾ���õ�ع���ʱ��a�ڷų�������Ϊ_________���õ�������ĵ缫��ӦʽΪ��____ ������һ��ʱ���3.2g������ȫ��Ӧ����CO2ʱ���� mol ���ӷ���ת�ơ�
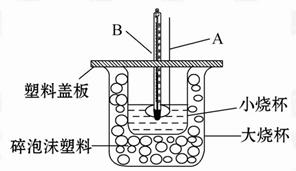
ijʵ��С�������50 mL 1.0 mol/L�����50 mL 1.1 mol/L ����������Һ����ͼװ���н����кͷ�Ӧ���ڴ��ձ��ײ�������ĭ����(��ֽ��)��ʹ�����С�ձ���������ձ�������ƽ��Ȼ�����ڴ�С�ձ�֮����������ĭ����(��ֽ��)�����ձ�������ĭ���ϰ�(��Ӳֽ��)���ǰ壬�ڰ��м俪����С�ף�����ʹ�¶ȼƺͻ��β��������ͨ����ͨ���ⶨ��Ӧ���������ų��������ɼ����к��ȡ��Իش��������⣺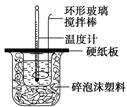
��1����ʵ�������Թ�����NaOH��ԭ��̲���˵��Ϊ��֤������ȫ���к͡����ʣ������ڷ�Ӧ������Ϊ�з�������������������ڷ�Ӧ�лӷ������õ��к��� (�ƫ����ƫС�����䡱)��
��2�����к��Ȳⶨʵ���д�����ˮϴ���¶ȼ��ϵ�����IJ��裬���˲������裬���õ��к��Ȼ� (�ƫ����ƫС�����䡱)��
��3�����õ�Ũ�ȵĴ�����NaOH��Һ��Ӧ�����õ��к��Ȼ� (�ƫ����ƫС�����䡱)����ԭ���� ��
��4����ʵ��С����������ʵ�飬ÿ��ȡ��Һ��50 mL������¼��ԭʼ����(���±�)��
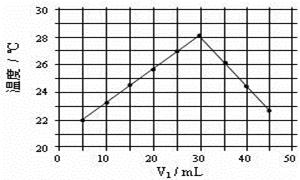
| ʵ����� | ��ʼ�¶�t1/�� | ��ֹ�¶�(t2)/�� | �²�(t2��t1)/�� | ||
| ���� | NaOH��Һ | ƽ��ֵ | |||
| 1 | 25.1 | 24.9 | 25.0 | 31.6 | 6.6 |
| 2 | 25.1 | 25.1 | 25.1 | 31.8 | 6.7 |
| 3 | 25.1 | 25.1 | 25.1 | 31.9 | 6.8 |
��֪���ᡢNaOH��Һ�ܶȽ���Ϊ1.00g/cm3���кͺ���Һ�ı�����c��4.18��10��3kJ/(g����)����÷�Ӧ���к���Ϊ��H�� �����ݼ�������д�����кͷ�Ӧ���Ȼ�ѧ����ʽ ��
��ҵ�������ڸ�¯�н��еģ���¯��������Ҫ��Ӧ�ǣ��� 2C����̿����O2����������2CO���� Fe2O3��3CO��2Fe��3CO�����������У��Խ�̿��ʵ��ʹ����ҪԶԶ���ڰ��ջ�ѧ����ʽ������������Ҫԭ����
| A��CO���� | B��CO������ʯ�Ӵ������ |
| C��������¯�ĸ߶Ȳ��� | D��CO��Fe2O3�ķ�Ӧ��һ���� |
������ѧ��Ӧ���ʵ���Ҫ������
| A������ | B���μӷ�Ӧ�����ʱ��������� |
| C����Ӧ���Ũ�� | D���¶ȡ�ѹǿ�Լ���Ӧ��ĽӴ��� |
��֪X��g��+3Y��g���T 2Z��g����H��0�����жԸ÷�Ӧ��˵������ȷ����
| A����S��0 |
| B���������Է����У��ҷ�Ӧ���ʺ�ƽ�ⳣ�����ϴ� |
| C����Ӧ������������������������ |
| D�����κ��¶��¶������Է����� |