��Ŀ����
ij�¶��£���һ�������0.1mol/L������Һ����μ���0.1mol/LNaOH��Һ����Һ��pOH��ע��pOH=-lgc(OH-)����pH�ı仯��ϵ��ͼ��ʾ��������˵������ȷ����
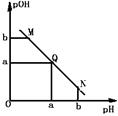

| A��M����ʾ��Һ��c(Na+)��c(CH3COO-)��c(OH-)��c(H+) |
| B��N����ʾ��Һ��c(CH3COO-)��c(Na+) |
| C��M���N����ʾ��Һ��ˮ�ĵ���̶���ͬ |
| D��Q������NaOH��Һ��������ڴ�����Һ����� |
C
����������⣺���ڴ��������ᣬ����̶Ⱥ�С������Ũ��Ҳ��С��M����Һ�ĵ���������������A����N����ʾ��ҺΪ���ԣ�������Һ����غ����жϳ���ʱc��Na������c��CH3COO��������B��������M���H��Ũ�ȵ���N���OH��Ũ�ȣ���ˮ�ĵ���̶�����������ͬ����������ˮ����̶���ͬ����C��ȷ��Q���pOH=pH����ҺΪ���ԣ������ߵ������Ϻ����ɴ����ƣ�ˮ���Լ��ԣ�������NaOH��Һ�����С�ڴ�����Һ���������D����ѡC��

��ϰ��ϵ�д�
 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
�����Ŀ
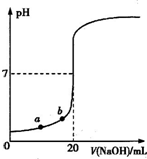

 H+ + OH-������������ȷ����
H+ + OH-������������ȷ����