��Ŀ����
��2013?����һģ�������й����ӷ���ʽ��д��ȷ�ģ�������
������A��������Һ�����ӵĻ�ԭ�ԣ�I-��Fe2+���жϷ�Ӧ������˳������Լ����ӷ���ʽ����д��
B������ǿ���������ԭ����������
C������NaHSO4��Һ��Ba��OH��2��Һ��Ӧ�����ԣ����ߵ����ʵ���֮��Ϊ2��1��
D�����ݵ��ʱ�����������ǽϻ��õĽ������ڵ�����������ʧ���ӣ��������۲�����Σ����������ӵõ��ӣ�Ȼ������ܷ�Ӧ=�����ĵ缫��Ӧ+�����ĵ缫��Ӧ��
B������ǿ���������ԭ����������
C������NaHSO4��Һ��Ba��OH��2��Һ��Ӧ�����ԣ����ߵ����ʵ���֮��Ϊ2��1��
D�����ݵ��ʱ�����������ǽϻ��õĽ������ڵ�����������ʧ���ӣ��������۲�����Σ����������ӵõ��ӣ�Ȼ������ܷ�Ӧ=�����ĵ缫��Ӧ+�����ĵ缫��Ӧ��
����⣺A������Һ�����ӵĻ�ԭ�ԣ�I-��Fe2+������Cl2������I-��Ȼ������Fe2+����1mol FeI2��Һ��ͨ���״����22.4L Cl2ʱ��ֻ��I-�����������ӷ���ʽΪ��2I-+Cl2�TI2+2Cl--����A����
B��CH3COOH�����Դ���H2CO3�����Բ�����H2CO3���Ʊ�CH3COOH����B����
C��NaHSO4��Һ��Ba��OH��2��Һ��Ӧ�����ԣ����ߵ����ʵ���֮��Ϊ2��1�����ӷ�ӦΪ2H++SO42-+Ba2++2OH-=BaSO4��+2H2O����C��ȷ��
D�����ʱ�������ĵ缫��Ӧ��2Ag++2e-=2Ag�������ĵ缫��Ӧ��Cu2++2e-=Cu�������ܷ�Ӧ��2Ag++Cu2+=2Ag+Cu����D����
��ѡC��
B��CH3COOH�����Դ���H2CO3�����Բ�����H2CO3���Ʊ�CH3COOH����B����
C��NaHSO4��Һ��Ba��OH��2��Һ��Ӧ�����ԣ����ߵ����ʵ���֮��Ϊ2��1�����ӷ�ӦΪ2H++SO42-+Ba2++2OH-=BaSO4��+2H2O����C��ȷ��
D�����ʱ�������ĵ缫��Ӧ��2Ag++2e-=2Ag�������ĵ缫��Ӧ��Cu2++2e-=Cu�������ܷ�Ӧ��2Ag++Cu2+=2Ag+Cu����D����
��ѡC��
���������⿼�����ӷ�Ӧ����ʽ��д����ȷ�����Ļ�ѧ��Ӧ�����ӷ�Ӧ����д�������ɽ���ѶȲ���

��ϰ��ϵ�д�
 ��У����ϵ�д�
��У����ϵ�д�
�����Ŀ
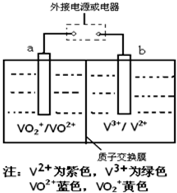 ��2013?����һģ��ij����ص�ԭ����ͼ��ʾ����Һ��c��H+��=2.0mol?L-1��������ΪSO42-��a��b��Ϊ���Ե缫�����ʱ�Ҳ���Һ��ɫ����ɫ��Ϊ��ɫ�����жԴ˵��������ȷ���ǣ�������
��2013?����һģ��ij����ص�ԭ����ͼ��ʾ����Һ��c��H+��=2.0mol?L-1��������ΪSO42-��a��b��Ϊ���Ե缫�����ʱ�Ҳ���Һ��ɫ����ɫ��Ϊ��ɫ�����жԴ˵��������ȷ���ǣ�������