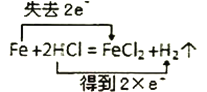
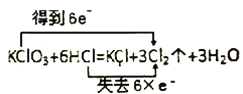
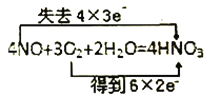
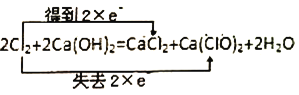��Ŀ����
����Ŀ�����л���ı�ʾ�������ֶ����������dz��õ��л���ı�ʾ������
��![]() ��CH3CH2CH(CH3)CH3��CH4 ��
��CH3CH2CH(CH3)CH3��CH4 ��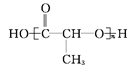 ��
��
�� ��
�� ��
��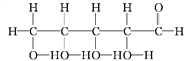
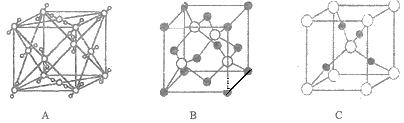
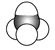
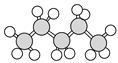
��1��������ʾ���������ڽṹ��ʽ����_______________(����ţ���ͬ)�����ڱ���ģ�͵���_______��
��2��д�����й����ŵ����ƣ�____________��____________��
��3��____________��____________��Ϊͬ���칹�塣
��1��д�������л���Ľṹ��ʽ��
��2��4����3�һ�����____________��
��2��2��ϩ____________��
��2��������������������
��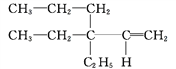 ____________��
____________��
��(CH3)3CCH(CH3)CH2CH3____________��
���𰸡��٢ڢۢܢ� �� �ǻ� ȩ�� �� �� 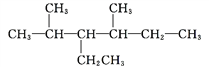
![]() 3��3���һ�1��ϩ�� 2��2��3��������
3��3���һ�1��ϩ�� 2��2��3��������
��������
��1���٢ڢۢܢ������л���Ľṹ��ʽ���������л���ı���ģ�ͣ��������л�������ģ�ͣ��������л���Ľṹʽ��
��2��ע������������֣���OH���ǻ���![]() ��ȩ����
��ȩ����
��3���ڢķ���ʽ����C5H12�����ṹ��ͬ������ͬ���칹�壻
��1����д����������д֧����2��4��������3���һ�����Ľṹ��ʽΪ ��2��2��ϩ��������д��C��C
��2��2��ϩ��������д��C��C![]() C��C��C������2��̼ԭ�����������������ԭ�Ӳ��룬���ṹ��ʽΪ
C��C��C������2��̼ԭ�����������������ԭ�Ӳ��룬���ṹ��ʽΪ![]() ��
��
��2���ٺ���̼̼˫�����̼������6��̼ԭ�ӣ�������3��3�����һ���1����ϩ��
�ڽ��ϲ��Ľṹ��ʽ��д�ɷֿ��Ľṹ��ʽ��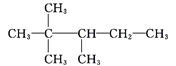 �����������2��2��3���������顣
�����������2��2��3���������顣

 ��������ܸ�ϰϵ�д�
��������ܸ�ϰϵ�д�����Ŀ��T Kʱ����2.0 L�����ܱ������г���1.0 mol COCl2����ӦCOCl2(g)![]() Cl2(g)��CO(g)������һ��ʱ���ﵽƽ�⡣��Ӧ�����вⶨ�IJ������ݼ��±���
Cl2(g)��CO(g)������һ��ʱ���ﵽƽ�⡣��Ӧ�����вⶨ�IJ������ݼ��±���
t / s | 0 | 2 | 4 | 6 | 8 |
n(Cl2) / mol | 0 | 0.16 | 0.19 | 0. 20 | 0.20 |
����˵����ȷ����( )
A. ��Ӧ��ǰ2 s ��ƽ������v(CO)=0.080mol��L-1��s-1
B. ���������������䣬�����¶ȣ�ƽ��ʱc(Cl2) =0.11mol��L-1����Ӧ�Ħ�H��0
C. T Kʱ��ʼ�������г���0.9 mol COCl2��0.10 mol Cl2��0.10 mol CO���ﵽƽ��ǰv��>v��
D. T Kʱ��ʼ�������г���1.0 mol Cl2��0.9 mol CO���ﵽƽ��ʱ��Cl2��ת����Ϊ80%
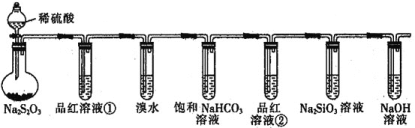
����Ŀ��ʵ������ijЩ�������ȡ���ռ���β������![]() ������ʵ��
������ʵ��![]() װ����ͼ��ʾ���ô�װ�ú��±����ṩ������������ʵ�飬������ѡ����
װ����ͼ��ʾ���ô�װ�ú��±����ṩ������������ʵ�飬������ѡ����![]()
ѡ�� | ���е����� | �����ռ������� | ���е����� |
|
A | Cu��ϡ���� | NO | NaOH��Һ | |
B | Ũ����� |
| NaOH��Һ | |
C | �������ƺ�Ũ���� |
| Ʒ����Һ | |
D | Ũ��ˮ��CaO |
| ��̪��Һ |
A.AB.BC.CD.D