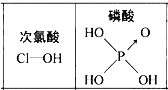
��Ŀ����
����Ŀ����������һ����ȻȾ�ϣ���ҵ�Ͽ���ʯ�͵��ѽ����ͨ����ͼ��Ӧ�Ƶã�
��֪��![]()
![]() CH3CHO+
CH3CHO+![]()
![]()
![]() +H2O
+H2O
��ش��������⣺
![]() �Լ�XΪ ______ ��
�Լ�XΪ ______ ��
![]() ���������Na��NaOH��
���������Na��NaOH��![]() �����ʵ����ֱ�Ϊ3mo1��2mol��1mol����E�Ľṹ��ʽΪ ______ ��
�����ʵ����ֱ�Ϊ3mo1��2mol��1mol����E�Ľṹ��ʽΪ ______ ��
![]() �������еĺ��������ų�������
�������еĺ��������ų�������![]() ��� ______
��� ______ ![]() ���
���![]() ��
��
![]() ��Ӧ
��Ӧ![]() �Ļ�ѧ����ʽΪ ______ ���䷴Ӧ������ ______ ��
�Ļ�ѧ����ʽΪ ______ ���䷴Ӧ������ ______ ��
![]() ������������G��ͬ���칹�干�� ______ �֣����к˴Ź�����������5��壬�������Ϊ2:2:2:1:1���� ______ ��
������������G��ͬ���칹�干�� ______ �֣����к˴Ź�����������5��壬�������Ϊ2:2:2:1:1���� ______ ��
�����ڷ������� �ڱ�����������ȡ���� ������![]() ��Һ������ɫ��Ӧ
��Һ������ɫ��Ӧ
![]() �����ȡ�����صķ���Ҳ�ܺϳ����ȩ(
�����ȡ�����صķ���Ҳ�ܺϳ����ȩ(![]() )��д���Ʊ����ȩ�����л���Ľṹ��ʽ ______ ��
)��д���Ʊ����ȩ�����л���Ľṹ��ʽ ______ ��
���𰸡�NaOH��ˮ��Һ 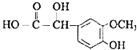 �ǻ����ʻ�
�ǻ����ʻ� ![]() ������Ӧ 9
������Ӧ 9 ![]()
![]() ��
��![]()
��������
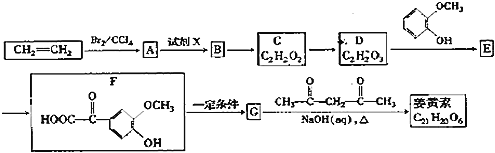
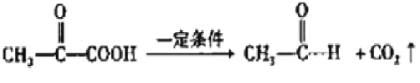
��ϩ���巢���ӳɷ�Ӧ����AΪBrCH2CH2Br����A��D��ϵ��ת����C��D����ʽ��֪��C��ȩ����������D����A����������ˮ��Һ�����������·���ˮ�ⷴӦ����BΪHOCH2CH2OH��B��������������CΪOHC-CHO��C�в���ȩ������������DΪOHC-COOH��1molE���������Na��NaOH��NaHCO3�����ʵ����ֱ�Ϊ3mo1��2mol��1mol�����F�Ľṹ��֪��D��ȩ�������ӳ�����EΪ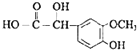 ��E�е�ȩ������������F��F��һ�������·�����Ϣi�����ȷ�Ӧ����GΪ
��E�е�ȩ������������F��F��һ�������·�����Ϣi�����ȷ�Ӧ����GΪ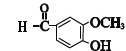 �������Ϣii�������صķ���ʽ����֪�����صĽṹ��ʽΪ��
�������Ϣii�������صķ���ʽ����֪�����صĽṹ��ʽΪ��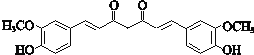 ��
��
(1)A��B����±������ˮ�ⷴӦ���Լ�XΪNaOH��ˮ��Һ��
�ʴ�Ϊ��NaOH��ˮ��Һ��
(2)E�Ľṹ��ʽΪ�� ��
��
�ʴ�Ϊ�� ��
��
(3)�����صĽṹ��ʽΪ�� ���������еĺ��������ų���������CH3O����У��ǻ����ʻ���
���������еĺ��������ų���������CH3O����У��ǻ����ʻ���
�ʴ�Ϊ���ǻ����ʻ���
(4)��ӦB��C�Ļ�ѧ����ʽΪ��![]() ������������Ӧ��
������������Ӧ��
�ʴ�Ϊ��![]() ��������Ӧ��
��������Ӧ��
(5)G(
![]() ��ͬ���칹����������������ڷ��������࣬�ڱ�����������ȡ������������FeCl3��Һ������ɫ��Ӧ��˵�����з��ǻ������Ա����ϳ����ǻ�֮�⣬��һȡ�������ܵĽṹ��-OOCCH3��-COOCH3��-CH2OOCH�������ڡ��䡢��3�֣����Է�������������ȩ��ͬ���칹�干��9�֣����к˴Ź�����������5��壬�������Ϊ2��2��2��1��1����
��ͬ���칹����������������ڷ��������࣬�ڱ�����������ȡ������������FeCl3��Һ������ɫ��Ӧ��˵�����з��ǻ������Ա����ϳ����ǻ�֮�⣬��һȡ�������ܵĽṹ��-OOCCH3��-COOCH3��-CH2OOCH�������ڡ��䡢��3�֣����Է�������������ȩ��ͬ���칹�干��9�֣����к˴Ź�����������5��壬�������Ϊ2��2��2��1��1����![]() ��
��
�ʴ�Ϊ��9��![]() ��
��
(6)�����ȡ�����صķ���Ҳ�ܺϳ����ȩ![]()
![]()
![]() ���Ʊ����ȩ�����л���Ľṹ��ʽΪ
���Ʊ����ȩ�����л���Ľṹ��ʽΪ![]() ��CH3CHO��
��CH3CHO��
�ʴ�Ϊ��![]() ��CH3CHO��
��CH3CHO��

����Ŀ��X��Y�� Z���ֿ������Σ��������ӷֱ���Ba2+��Cu2+��Ag+�е�ijһ�֣������ӷֱ���Cl-��SO42����NO3���е�ijһ�֡���������ʵ�飺
�ٽ������θ�ȡ�������ֱ�����ʢ��5 mL����ˮ����֧�Թ��У�ֻ��X����Һ����ɫ��
�ڷֱ�����֧�Թ��м���2 mLϡ���ᣬ����Y����Һ�в�����ɫ������Z����Һ����������
(1)����������ʵ���ƶ��������εĻ�ѧʽ��X______��Y______��Z______��
(2)����������������Ba2+��Cu2+��Ag+��Ӧ���ζ�Ϊ�����Σ������������Һ��ѡ������Լ�����������������һ�������롣������ͼ���£�

���������ܽ��Ա�Ϊ
Ba2+ | Cu2+ | Ag+ | |
Cl- | �� | �� | �� |
CO32�� | �� | �� | �� |
SO42�� | �� | �� | |
S2- | �� | �� | �� |
�ٳ���1�Ļ�ѧʽΪ_________________��
��д�������Һ���Լ�A��Ӧ�����ӷ���ʽ��______________��
����CΪNa2S��������B����������ͬ�����Լ�B��������____________��