��Ŀ����
��Դ����������������ٵ��ش���⣬�״���һ�ֿ�������Դ�����п�����Ӧ�õĹ���ǰ�����о��״�������Ҫ���塣
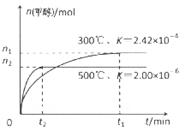
��1����CO�ϳɼ״��ķ�ӦΪ��CO(g)+2H2(g) CH3OH(g)���ݻ�Ϊ1L�����ܱ������зֱ����1molCO��2molH2��ʵ���ü״������ʵ������¶ȡ�ʱ��Ĺ�ϵ������ͼ��ʾ���������Ӧ�ġ�H_______0�����������������=�������жϵ�������______��
CH3OH(g)���ݻ�Ϊ1L�����ܱ������зֱ����1molCO��2molH2��ʵ���ü״������ʵ������¶ȡ�ʱ��Ĺ�ϵ������ͼ��ʾ���������Ӧ�ġ�H_______0�����������������=�������жϵ�������______��
��2�����ù�ҵ�����е�CO2����ȡ�״����䷴ӦΪ��CO2+3H2 CH3OH+H2O��
CH3OH+H2O��
�ٳ��³�ѹ����֪���з�Ӧ�������仯����ͼ��ʾ��

�ɶ�����̼�������Ʊ��״����Ȼ�ѧ����ʽΪ_______��
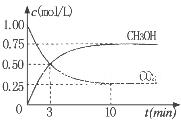
��Ϊ̽����CO2����ȼ�ϼ״��ķ�Ӧԭ�����ֽ�������ʵ�飺��һ���º����ܱ������У�����1molCO2��3molH2������������Ӧ�����CO2����CH3OH(g)��Ũ����ʱ��仯��ͼ��ʾ���ӷ�Ӧ��ʼ��ƽ�⣬v(H2)="_______" �����¶��µ�ƽ�ⳣ����ֵK=______����ʹƽ����ϵ��n(CH3OH)/n(CO2))����Ĵ�ʩ��_______����дһ������
��3����ҵ�����ü״��Ʊ������ij��÷��������֡�
�ټ״���������������Ҫ��ӦΪ��CH3OH(g) CO(g)+2H2(g)�����ݻ�Ϊ2.0L���ܱ������г���0. 60 molCH3OH(g)����ϵѹǿΪP1����һ�������´ﵽƽ��ʱ����ϵѹǿΪP2����P2/P1 =2.2�����������CH3OH ��ƽ��ת����Ϊ______ ��
CO(g)+2H2(g)�����ݻ�Ϊ2.0L���ܱ������г���0. 60 molCH3OH(g)����ϵѹǿΪP1����һ�������´ﵽƽ��ʱ����ϵѹǿΪP2����P2/P1 =2.2�����������CH3OH ��ƽ��ת����Ϊ______ ��
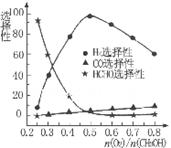
�ڼ״���������������һ���¶�����Ag/CeO2��ZnOΪ����ʱԭ���������Է�Ӧ��ѡ���ԣ�ѡ����Խ��ʾ���ɵĸ�����Խ�ࣩӰ���ϵ��ͼ��ʾ����n(O2)��n(CH3OH) =0.25ʱ��CH3OH��O2��������Ҫ��Ӧ����ʽΪ______ �����Ʊ�H2��ʱ��ÿ���n(O2))/n(CH3OH)=______��
��1����CO�ϳɼ״��ķ�ӦΪ��CO(g)+2H2(g)
 CH3OH(g)���ݻ�Ϊ1L�����ܱ������зֱ����1molCO��2molH2��ʵ���ü״������ʵ������¶ȡ�ʱ��Ĺ�ϵ������ͼ��ʾ���������Ӧ�ġ�H_______0�����������������=�������жϵ�������______��
CH3OH(g)���ݻ�Ϊ1L�����ܱ������зֱ����1molCO��2molH2��ʵ���ü״������ʵ������¶ȡ�ʱ��Ĺ�ϵ������ͼ��ʾ���������Ӧ�ġ�H_______0�����������������=�������жϵ�������______��
��2�����ù�ҵ�����е�CO2����ȡ�״����䷴ӦΪ��CO2+3H2
 CH3OH+H2O��
CH3OH+H2O���ٳ��³�ѹ����֪���з�Ӧ�������仯����ͼ��ʾ��

�ɶ�����̼�������Ʊ��״����Ȼ�ѧ����ʽΪ_______��
��Ϊ̽����CO2����ȼ�ϼ״��ķ�Ӧԭ�����ֽ�������ʵ�飺��һ���º����ܱ������У�����1molCO2��3molH2������������Ӧ�����CO2����CH3OH(g)��Ũ����ʱ��仯��ͼ��ʾ���ӷ�Ӧ��ʼ��ƽ�⣬v(H2)="_______" �����¶��µ�ƽ�ⳣ����ֵK=______����ʹƽ����ϵ��n(CH3OH)/n(CO2))����Ĵ�ʩ��_______����дһ������

��3����ҵ�����ü״��Ʊ������ij��÷��������֡�
�ټ״���������������Ҫ��ӦΪ��CH3OH(g)
 CO(g)+2H2(g)�����ݻ�Ϊ2.0L���ܱ������г���0. 60 molCH3OH(g)����ϵѹǿΪP1����һ�������´ﵽƽ��ʱ����ϵѹǿΪP2����P2/P1 =2.2�����������CH3OH ��ƽ��ת����Ϊ______ ��
CO(g)+2H2(g)�����ݻ�Ϊ2.0L���ܱ������г���0. 60 molCH3OH(g)����ϵѹǿΪP1����һ�������´ﵽƽ��ʱ����ϵѹǿΪP2����P2/P1 =2.2�����������CH3OH ��ƽ��ת����Ϊ______ ���ڼ״���������������һ���¶�����Ag/CeO2��ZnOΪ����ʱԭ���������Է�Ӧ��ѡ���ԣ�ѡ����Խ��ʾ���ɵĸ�����Խ�ࣩӰ���ϵ��ͼ��ʾ����n(O2)��n(CH3OH) =0.25ʱ��CH3OH��O2��������Ҫ��Ӧ����ʽΪ______ �����Ʊ�H2��ʱ��ÿ���n(O2))/n(CH3OH)=______��

��1�����¶����ߣ�ƽ��ʱ�״��������٣�ƽ�������ƶ���������Ӧ���ȣ����¶����ߣ�ƽ�ⳣ����С��ƽ�������ƶ���������Ӧ���ȣ�����2���� CO2(g)+3H2(g)=CH3OH(l)+H2O(l) ��H="-50KJ/mol." ��0.225mol/(L��min) 5.3 �����¶ȣ����ѹ������H2������H2O��������ϵ�з���ȣ�����3�� ��60�� ��2CH3OH+O2 ="2HCHO+" 2H2O 0. 5
�����������1������Ӧ�ﵽƽ������������¶ȣ�n(CH3OH)��С��ƽ��ʱCH3OH�ĺ������ͣ�˵�������¶ȣ���ѧƽ�����淴Ӧ�����ƶ�������ƽ���ƶ�ԭ���������¶ȣ���ѧƽ�������ȷ�Ӧ�����ƶ����淴Ӧ���������ȷ�Ӧ����������Ӧ�Ƿ��ȷ�Ӧ���ʡ�H��0.��2�� ����ͼһ��֪��CO2(g)+H2(g)=CO(g)+H2O(l) ��H="41KJ/mol," ��ͼ����֪��CO(g)+2H2(g)
 CH3OH(g) ��H= -91KJ/mol.����ʽ��ӿɵã�CO2(g) +3H2(g)=CH3OH(l)+H2O(l) ��H="-50KJ/mol." �� V(CO2)=" (1.00-0.25)" mol/L��10min=" 0.075mol/(l��min)." V(H2):V(CO2)=3:1,����V(H2)="3" V(CO2)=" 0.225mol/(L��min)" . �ڸ��¶��µ�ƽ�ⳣ����ֵ
CH3OH(g) ��H= -91KJ/mol.����ʽ��ӿɵã�CO2(g) +3H2(g)=CH3OH(l)+H2O(l) ��H="-50KJ/mol." �� V(CO2)=" (1.00-0.25)" mol/L��10min=" 0.075mol/(l��min)." V(H2):V(CO2)=3:1,����V(H2)="3" V(CO2)=" 0.225mol/(L��min)" . �ڸ��¶��µ�ƽ�ⳣ����ֵ ���ڷ�Ӧ CO2(g) +3H2(g)= CH3OH(l)+H2O(l) ��H=-50KJ/mol.������Ӧ��һ�����ȷ�Ӧ�����Խ����¶���ʹƽ����ϵ��n(CH3OH)/n(CO2)������������ѹ������H2������ˮ�����ӻ�����з�������ȴ�ʩҲ��ʹƽ����ϵ��n(CH3OH)/n(CO2))����3����Ӧ��ʼʱn(CH3OH)=0.6mol,n(CO)=0mol,n(H2)=0mol.���跴Ӧ������CH3OH�ı�����ʵ���ΪX����ﵽƽ��ʱ�����ʵ����ʵ���Ϊn(CH3OH)=" (0.6-X)mol," n(CO) ="Xmol" n(H2)=2Xmol,��������̶����ܱ������е����巴Ӧ��˵����Ӧǰ���ѹǿ�ȵ������ǵ����ʵ����ıȡ�����(0.6+2X)��0.6=2.2,���X=0.36.����CH3OH��ƽ��ת����Ϊ0.36��0.6��100��=60��������ͼ��֪��n(O2)��n(CH3OH) =0.25ʱ�õ��IJ����Ǽ�ȩ��CH3OH��O2��������Ҫ��Ӧ����ʽΪ2CH3OH +O2="2HCHO+" 2H2O�����Ʊ�H2ʱ������n(O2)��n(CH3OH) =0.5ʱѡ������ߣ�������ÿ���n(O2))/n(CH3OH)= 0.5��
���ڷ�Ӧ CO2(g) +3H2(g)= CH3OH(l)+H2O(l) ��H=-50KJ/mol.������Ӧ��һ�����ȷ�Ӧ�����Խ����¶���ʹƽ����ϵ��n(CH3OH)/n(CO2)������������ѹ������H2������ˮ�����ӻ�����з�������ȴ�ʩҲ��ʹƽ����ϵ��n(CH3OH)/n(CO2))����3����Ӧ��ʼʱn(CH3OH)=0.6mol,n(CO)=0mol,n(H2)=0mol.���跴Ӧ������CH3OH�ı�����ʵ���ΪX����ﵽƽ��ʱ�����ʵ����ʵ���Ϊn(CH3OH)=" (0.6-X)mol," n(CO) ="Xmol" n(H2)=2Xmol,��������̶����ܱ������е����巴Ӧ��˵����Ӧǰ���ѹǿ�ȵ������ǵ����ʵ����ıȡ�����(0.6+2X)��0.6=2.2,���X=0.36.����CH3OH��ƽ��ת����Ϊ0.36��0.6��100��=60��������ͼ��֪��n(O2)��n(CH3OH) =0.25ʱ�õ��IJ����Ǽ�ȩ��CH3OH��O2��������Ҫ��Ӧ����ʽΪ2CH3OH +O2="2HCHO+" 2H2O�����Ʊ�H2ʱ������n(O2)��n(CH3OH) =0.5ʱѡ������ߣ�������ÿ���n(O2))/n(CH3OH)= 0.5��
��ϰ��ϵ�д�
 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
�����Ŀ

 2C(��) ��H��0�����и�ͼ����ȷ����
2C(��) ��H��0�����и�ͼ����ȷ����
 CO(NH2)2(s)+H2O(g)
CO(NH2)2(s)+H2O(g)  2NH3(g) ��92.4 kJ������ʱ����ϵ�и�����Ũ����ʱ��仯��������ͼʾ������˵���������
2NH3(g) ��92.4 kJ������ʱ����ϵ�и�����Ũ����ʱ��仯��������ͼʾ������˵���������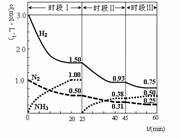
 2C(g)(����Ӧ����)������ͼ����ȷ����
2C(g)(����Ӧ����)������ͼ����ȷ����
 2C��g��+ D��s�� ��5 min��ﵽƽ�⡣ƽ��ʱAΪ1.6mol���ų�������ΪQ����t0ʱ�̣�����ƽ����ϵ�з�����ķ�֮һ�Ļ�����壬��ƽ����ϵ��c(A)Ϊ0.6mol��L��
2C��g��+ D��s�� ��5 min��ﵽƽ�⡣ƽ��ʱAΪ1.6mol���ų�������ΪQ����t0ʱ�̣�����ƽ����ϵ�з�����ķ�֮һ�Ļ�����壬��ƽ����ϵ��c(A)Ϊ0.6mol��L�� 4NO2(g)+O2(g) ��H>0
4NO2(g)+O2(g) ��H>0 �����CH4��ƽ��ת�������¶ȼ�ѹǿ�Ĺ�ϵ��ͼ�������й�˵��һ����ȷ����
�����CH4��ƽ��ת�������¶ȼ�ѹǿ�Ĺ�ϵ��ͼ�������й�˵��һ����ȷ����