��Ŀ����
15��ʵ��������0.1mol•L-1��BaCl2��Һ�ɷ������ν��У���һ�Σ���������ƽ��ȡ5.2g��ˮBaCl2���壮�ڶ��Σ��ܽ�����0.1mol•L-1��BaCl2��Һ����һ�β��������¼�����
A�������벦��0.2g����
B�������벦����0������
C������ƽ���ߵ������ϸ���һ�Ÿɾ��ĵ���������ֽ��������ƽ���ߵ���ĸʹ��ƽƽ�⣻
D��ȡ��ҩƷ��������Ż�������ڣ�
E���������������Ӿ�������ƽƽ�⣻
F���������Ϸ���5g���룮
��1������ȷ�IJ���˳���ǣ�����ţ���B��C��F��A��E��D��B
��2����E�����У�ֻȱ��������ʱ���������������������ʹ�������������ձ��У�
��3���ڶ��β�����Ӧ�Ƚ�5.2g BaCl2����������ˮ�ܽ⣬�ܽ������ʹ�õ���Ҫ�������ձ�����������Ȼ����Һת��250mL����ƿ�У��پ�ϴ�ӡ����ݡ�ҡ�Ⱥɵõ�0.1 mol•L-1 BaCl2��Һ��
��4�����в���ʹ���Ƶ�BaCl2��ҺŨ��ƫ�͵���AC��
A����������������ϣ�BaCl2���������Ͻ��г���
B��ѡ�õ�����ƿ������������ˮ
C������ҡ�Ⱥ�Һ���½����ּ�ˮ���̶���
D���������ƹ����У�����ƿ����
��5��100mL����ƿ��ʢ��100mL 0.1010mol•L-1��BaCl2��Һ����������ϡ�ͳ�Ũ��Ϊ0.100mol•L-1��BaCl2��Һ����ѡ�õ������У�10mL��Ͳ��1mL��Һ�ܣ���ȷ��ȡ0.1mL��1.00mL��Һ������ʽ�ζ��ܡ���ͷ�ιܣ���IJ�����������1mL��Һ���Ƴ�0.99mL��0.1010mol•L-1��BaCl2��Һ��Ȼ���ý�ͷ�ι���ʣ�µ�99.01mL 0.1010mol•L-1��BaCl2��Һ�м�ˮ���̶��ߣ��������Ƶ�0.100mol•L-1��BaCl2��Һ��
���� ��1����������ƽ��ȡ5.2g��ˮBaCl2����ʱ��Ӧ�ȵ��㣬����Ҫ������ҩƷ������Ϊ5.2g��ѡ��5g�������0.2g���룬Ӧע��ҩƷӦ����ֽ�ϳ������ݴ˷�����
��2����������ҩƷʱ�����Ҫ����������ҩƷ��Ӧ��������ʹ�������������ձ��У�
��3���������Ʋ����Ǽ��㡢�������ܽ⡢��Һ��ϴ�ӡ����ݡ�ҡ�ȡ�װƿ�����������������Ҫ��������
��4�������������������ʵ����ʵ�������Һ�������Ӱ�죬����c=$\frac{n}{V}$������������
��5����������100mL0.100mol•L-1��BaCl2��Һ�������0.1010mol•L-1��BaCl2��Һ�����ΪVmL��Ȼ�������Һϡ�Ͷ���CŨVŨ=CϡVϡ��������������Һ�������Ȼ������Һ���Ƴ�һ�������0.1010mol•L-1��BaCl2��Һ��Ȼ���ý�ͷ�ι���ʣ�µ�0.1010mol•L-1��BaCl2��Һ�м�ˮ���̶��ߣ��������Ƶ�0.100mol•L-1��BaCl2��Һ��
��� �⣺��1����ƽ����ȷʹ�ò��裺a����ƽ�ŵ�ˮƽ̨�ϣ������Ƶ������˵���̶ȣ�
b������ƽ��ƽ����ĸʹָ��ָ���ֶ��̵����룬������ƫת�ĸ�����ͬ��
c���������ƽ�����̣����������ƽ�����̣������������ڱ���ϵ�λ�ã�ֱ�������ָ�ƽ�⣮Ҫ�����Ӽ�ȡ���룬Ҫ������ţ�Ҫ�Ӵ�С��
d���������=���������+�����Ӧ�Ŀ̶ȣ�
e�������ģ�������ȷ��˳��Ϊ��BCFAEDB��
�ʴ�Ϊ��BCFAEDB��
��2����������ҩƷʱ������ҩƷֻȱ��������ʱ��Ӧ��������ʹ�������������ձ��У�
�ʴ�Ϊ����������ʹ�������������ձ��У�
��3������һ�����ʵ���Ũ����Һ�IJ������裺���㡢�������ܽ⡢��Һ��ϴ�ӡ����ݡ�ҡ�ȣ��ܽ�ʱ������Ӧ�����ձ��У��ò��������Ͻ��裻
�ʴ�Ϊ���ձ�����������250mL����ƿ��ϴ�ӣ����ݣ�ҡ�ȣ�
��4��A����������������ϣ�BaCl2���������Ͻ��г��������³�ȡ������ƫС�����ʵ����ʵ���ƫС����Һ��Ũ��ƫ�ͣ���Aѡ��
B��ѡ�õ�����ƿ������������ˮ�������ʵ����ʵ�������Һ��������������Ӱ�죬��Һ��Ũ�Ȳ���Ӱ�죬��B��ѡ��
C������ҡ�Ⱥ�Һ���½����ּ�ˮ���̶��ߣ�������Һ�����ƫ����Һ��Ũ��ƫ�ͣ���Cѡ��
D���������ƹ����У�����ƿ�����ᵼ����Һ��ϲ����ȣ���Һ���������ƫ���ƫС����Һ��Ũ�ȿ���ƫ�ͻ�ƫ�ߣ���D��ѡ��
��ѡ��AC��
��5����������100mL0.100mol•L-1��BaCl2��Һ�������0.1010mol•L-1��BaCl2��Һ�����ΪVmL��������Һϡ�Ͷ���CŨVŨ=CϡVϡ��֪��
0.1010mol•L-1��VmL=100mL��0.100mol•L-1
���V=99.01mL������1mL��Һ���Ƴ�0.99mL��0.1010mol•L-1��BaCl2��Һ��Ȼ���ý�ͷ�ι���ʣ�µ�99.01mL 0.1010mol•L-1��BaCl2��Һ�м�ˮ���̶��ߣ��������Ƶ�0.100mol•L-1��BaCl2��Һ��
�ʴ�Ϊ����1mL��Һ���Ƴ�0.99mL��0.1010mol•L-1��BaCl2��Һ��Ȼ���ý�ͷ�ι���ʣ�µ�99.01mL 0.1010mol•L-1��BaCl2��Һ�м�ˮ���̶��ߣ��������Ƶ�0.100mol•L-1��BaCl2��Һ��
���� ���⿼��������һ�����ʵ���Ũ����Һ����Ŀ�ѶȲ����ؿ���ѧ����ʵ��������������ճ̶ȣ���ȷʵ��ԭ���ͻ��������ǽ���ؼ���ע�����֪ʶ�Ļ��ۣ�

 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�| A�� | ��NaOH��Һ��ȥCO2�л��е�HCl���� | |
| B�� | ��������ͭ�۳�ȥCu��NO3��2��Һ�е�AgNO3���� | |
| C�� | ���в���ֶ�ֻ���н���Ԫ�� | |
| D�� | ���Ҵ��ӵ�ˮ����ȡ�� |
| A�� | 11.2LO2��03��ɵĻ�����庬��ԭ����ΪNA | |
| B�� | ���³�ѹ�£�1.7gNH3���еĵ�����ĿΪNA | |
| C�� | .0.1mol/LNa2SO4��Һ�к���Na+�ĸ���Ϊ0.2NA | |
| D�� | ��״���£�22.4LNO2��������H2O��ַ�Ӧ��ת�Ƶ�����ΪNA�� |
| A�� | ������ĵ���ʽ��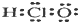 | B�� | �Ȼ��Ƶķ���ʽ��NaCl | ||
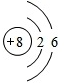
| C�� | ������̼�ı���ģ�ͣ� | D�� | Cl-�Ľṹʾ��ͼ��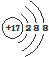 |

| A�� | X��NH3��Y��KCl��Һ | B�� | X��SO2��Y��NaOH | ||
| C�� | X��H2��Y��Ũ���� | D�� | X��CO2��Y��KOH |
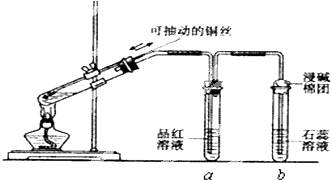
 ��
��