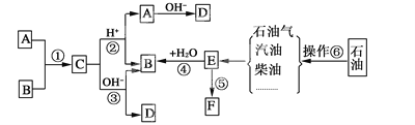
��Ŀ����
����Ŀ��X��Y��T��Q��Z ����Ԫ�أ�λ��Ԫ�����ڱ�ǰ�����ڣ�Ԫ�ص����ʻ�ṹ��Ϣ�����
Ԫ�� | ���ʻ�ṹ��Ϣ |
X | ����Ϊ˫ԭ�ӷ��ӣ������к���3�Թ��õ��Ӷԣ������µ������������ȶ�������ԭ�ӽϻ��� |
Y | ��̬ԭ�ӵ�3p�������4������ |
T | ԭ�Ӻ���s���������������p������������������ں�������Ԫ�أ����䵥���dz�������ȼ�� |
Q | ������������Ԫ����ԭ�Ӱ뾶��С |
Z | ��̬ԭ�ӵ�2��������M����ȫ���������� |
�����������Ϣ�ش��������⣺
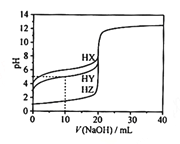
��1��д��X3����һ�ֵȵ�����Ļ�ѧʽ___��
��2��д��TԪ�ػ�̬ԭ�ӵĺ�������Ų�ͼ___��
��3��Ԫ��X��T�ĵ縺����ȣ�___��С����Ԫ�����ƣ���Ԫ��X�ĵ�һ��������T��Ƚϣ�T��___�����С������
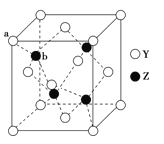
��4����ZԪ�������ڱ���λ��__����Z���ʾ�����Zԭ������ά�ռ���Ķѻ���ʽΪ___�ѻ���
��Z���Ȼ����백ˮ��Ӧ���γ������[Z��NH3��4��H2O��2]Cl2������������ʱ������ʧȥ�����е�������___��д��ѧʽ����
��5��Ԫ��X��Q���γɻ�����XQ3�����ݼ۲���ӶԻ��������ж�XQ3�Ŀռ乹��Ϊ___��������Xԭ�ӵ��ӻ���ʽΪ___�ӻ���
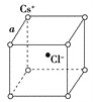
��6��Y��Z���γɻ����ᄃ��ľ�����ͼ��ʾ���û�����Ļ�ѧʽΪ___��
���𰸡�CO2 ![]() �� С ds �������ܶѻ� H2O ������ sp3 ZnS
�� С ds �������ܶѻ� H2O ������ sp3 ZnS
��������
X�γɵĵ���Ϊ˫ԭ�ӷ��ӣ������к���3�Թ��õ��Ӷԣ������µ������������ȶ�������ԭ�ӽϻ��ã���XΪNԪ�أ���̬ԭ�ӵ�3p�������4�����ӣ������Ų�ʽΪ1s22s22p63s23p4,YΪSԪ�أ�Tԭ�Ӻ���s���������������p������������������ں�������Ԫ�أ����䵥���dz�������ȼ����ΪOԪ�أ�QΪ������������Ԫ����ԭ�Ӱ뾶��СΪClԪ�أ�Z��̬ԭ�ӵ�2��������M����ȫ���������ӣ�ΪZnԪ�أ��ݴ˻ش����⣻
�ɷ�����֪XΪNԪ����YΪSԪ�أ�TΪOԪ����QΪClԪ�أ�ZΪZnԪ����
��1��X3��ΪN3������N3����ԭ�Ӹ�����ͬ���۵�������ͬ��һ�ֵȵ�����ΪCO2��
��2��TΪOԪ�أ���̬��ԭ�ӵ�����������ԳɶԵ��ӣ�2���ɵ����ӣ���������Ų�ͼΪ![]() ��
��
��3��XΪNԪ�أ�TΪOԪ����ͬ���ڴ������ҵ縺������ǿ����һ�����������ʵ��ĵ縺�Խ�С�����ڵ�ԭ�������p���Ϊ������ṹ����һ�����ܽ���ԭ�Ӵ��ĵ�һ�����ܽϴ�
��4����ZΪZnԪ�أ������ڱ���λ��ds��������ά�ռ���Ķѻ���ʽΪ�������ܶѻ���
����λԭ�ӵ縺��Խ���������ӵ�����Խǿ���������ӶԺ�����Ԫ����ϵ�������Խ�����γɵ���λ��Խ����������ʧȥ���縺��O>N���������ȣ�����ʧȥ�������е�������H2O��
��5��Ԫ�ص����ȿ��г�NCl3�����ݼ۲���ӶԻ������ۿ�֪���÷���������ԭ�Ӽ۲���ӶԸ�����4���Һ���һ���µ��Ӷԣ����Կռ乹��Ϊ�����Σ������е�ԭ�ӵ��ӻ���ʽΪs p3�ӻ���
��6��YΪSԪ�أ�ZΪZnԪ����S2-λ�ڶ�������ģ�Zn2+λ���ڲ�������λ�ڶ��㣬ͬΪ8�����������У��˷�֮һ���ڸþ�����λ�����ϣ�ͬΪ2�����������У�����֮һ���ڸþ�����λ���ڲ������ڸþ���������S2-����=![]() =4��Zn2+����=4��������Ϊ1:1�����Ի�ѧʽΪZnS��
=4��Zn2+����=4��������Ϊ1:1�����Ի�ѧʽΪZnS��

 ���Ͱ�ͨ�������Сѧ��ʱͬ�����ϵ�д�
���Ͱ�ͨ�������Сѧ��ʱͬ�����ϵ�д� ���Ͱ�ͨ������ϵ�д�
���Ͱ�ͨ������ϵ�д� �ٷ�ѧ����ҵ��������ϵ�д�
�ٷ�ѧ����ҵ��������ϵ�д�