��Ŀ����
��7�֣�2010��8��7�ո������������ش���ʯ���ֺ���ר��ָ����Ϊ��ֹ���ֹ������߷��������ú�����������������Ի���������
��1���������������Ŀǰ�����Ϲ��ϵĵ��Ĵ���Ч�����Ĺ�����������������KClO3��Һ��H2SO4��������Na2SO3��Һ��Ӧ�Ƶá���д���÷�Ӧ�����ӷ���ʽ��
��2����̼������һ���ж���;��������ϵ��̬Ư������ѧʽ�ɱ�ʾΪNa2CO3��3H2O2��������Na2CO3��H2O2��˫�����ʡ���̼�������������ʾ��ᷢ����ѧ��Ӧ��ʧЧ�����й�̼����ֻ��������������������������ţ���
��3��Ư����������(NaClO2)�ڳ������ںڰ����ɱ���һ�꣬������ȶ��ɷֽ⣬��Ӧ�����ӷ���ʽΪ��HClO2��ClO2��+H++Cl-+H2O(δ��ƽ)����1 mol HClO2�����ֽⷴӦʱ��ת�Ƶĵ�����������������
��4�����ʵı���������ָ���ȶ��ĵ�������1molָ������ʱ����ЧӦ����H2O(l)��������Ϊ-285.8 kJ��mol-1�����Ȼ�ѧ����ʽ��ʾΪ H2(g)+1/2O2(g)=H2O(l)����H= -285.8 kJ��mol-1�����֪ClO2�ı���������+102.5kJ��mol-1����÷�Ӧ���Ȼ�ѧ����ʽ��ʾΪ________________________________��
��1���������������Ŀǰ�����Ϲ��ϵĵ��Ĵ���Ч�����Ĺ�����������������KClO3��Һ��H2SO4��������Na2SO3��Һ��Ӧ�Ƶá���д���÷�Ӧ�����ӷ���ʽ��
��2����̼������һ���ж���;��������ϵ��̬Ư������ѧʽ�ɱ�ʾΪNa2CO3��3H2O2��������Na2CO3��H2O2��˫�����ʡ���̼�������������ʾ��ᷢ����ѧ��Ӧ��ʧЧ�����й�̼����ֻ��������������������������ţ���
| A��MnO2 | B��KMn O4��Һ O4��Һ | C��ϡ���� | D��Na2SO3��Һ |
��4�����ʵı���������ָ���ȶ��ĵ�������1molָ������ʱ����ЧӦ����H2O(l)��������Ϊ-285.8 kJ��mol-1�����Ȼ�ѧ����ʽ��ʾΪ H2(g)+1/2O2(g)=H2O(l)����H= -285.8 kJ��mol-1�����֪ClO2�ı���������+102.5kJ��mol-1����÷�Ӧ���Ȼ�ѧ����ʽ��ʾΪ________________________________��
��7�֣�
��1��2ClO3-��SO32-��2H+=2ClO2����SO42-��H2O ��2�֣�
��2��D�� ��1�֣�
��3��4.816��1023����0.8NA�� ��2�֣�
��4��Cl2(g)+2O2(g)=2ClO2(g)����H="+205.0" kJ��mol-1 ��2�֣�
��

��ϰ��ϵ�д�
 ������ĩ��ϰ��ѵ��ϵ�д�
������ĩ��ϰ��ѵ��ϵ�д� С��ʿ��ĩ����100��ϵ�д�
С��ʿ��ĩ����100��ϵ�д�
�����Ŀ
 ��
�� �ۣ�ά����B2 �ܣ�ά����C
�ۣ�ά����B2 �ܣ�ά����C ��һ�����ֲ�����ʯӢ��������Ҫ�ɷ��� ���л��������ۼ���ϩ�������ʵ���ϲ��ǹ����β��ϣ�����һ�� ����ѡ�������ά�������ϡ����л��������ɼ���ϩ�������һ��������ͨ�� ��Ӧ�ϳɵ��л��߷��Ӳ��ϡ�
��һ�����ֲ�����ʯӢ��������Ҫ�ɷ��� ���л��������ۼ���ϩ�������ʵ���ϲ��ǹ����β��ϣ�����һ�� ����ѡ�������ά�������ϡ����л��������ɼ���ϩ�������һ��������ͨ�� ��Ӧ�ϳɵ��л��߷��Ӳ��ϡ� OTC������ʾ_____________________������R������ʾ________ __������ƽʱ���Լ���ҩ����ҩ�簢˾ƥ����������ҩ__________________��
OTC������ʾ_____________________������R������ʾ________ __������ƽʱ���Լ���ҩ����ҩ�簢˾ƥ����������ҩ__________________��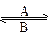 Fe3+��ת����A��Fe2+��__________��B��Fe3+��__________����
Fe3+��ת����A��Fe2+��__________��B��Fe3+��__________����