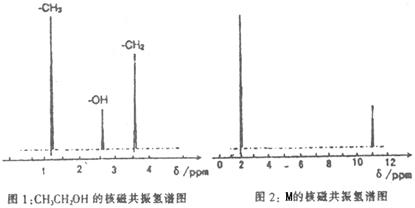
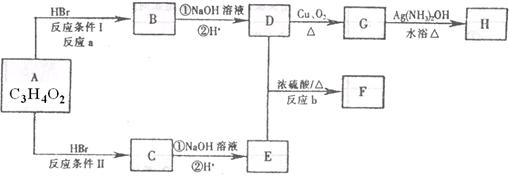
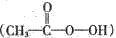��Ŀ����
�����г���ҩƷ�ܶ࣬�磺�ٵ�ơ�����Ƽ����ù�ء��ܰ�˾ƥ�֡���������ע��Һ����θ��ƽ�����������������۵ȣ���
(1)�������������ڿ����ص���_____����д��ţ���ͬ��������ֹ��ƽ�����õ���Ȼҩ������������
θ��ƽ�ɷ���θ����ڹ��࣬�䷢�ӹ�Чʱ�����ӷ���ʽΪ������������������������
(2)��Ѫ�Dz�֢����ʱ����������ˮ�ɿ��ټ�Ч����������ΪӪ�����������������������ڷ�������Ҫ��Ӧ��_________����д��ĸ����ͬ����
(1)�������������ڿ����ص���_____����д��ţ���ͬ��������ֹ��ƽ�����õ���Ȼҩ������������
θ��ƽ�ɷ���θ����ڹ��࣬�䷢�ӹ�Чʱ�����ӷ���ʽΪ������������������������
(2)��Ѫ�Dz�֢����ʱ����������ˮ�ɿ��ټ�Ч����������ΪӪ�����������������������ڷ�������Ҫ��Ӧ��_________����д��ĸ����ͬ����
| A���ӳɷ�Ӧ | B��ȡ����Ӧ | C��������Ӧ | D���ۺϷ�Ӧ |
��1���ۡ������ڡ�Al(OH)3+3H+=Al3++H2O��������2��C
��

��ϰ��ϵ�д�
 ӥ�ɽ̸��νӽ̲ĺӱ�����������ϵ�д�
ӥ�ɽ̸��νӽ̲ĺӱ�����������ϵ�д�
�����Ŀ
 O4��Һ
O4��Һ