��Ŀ����
��1������Ϊԭ���Ʊ�AgNO3�Ĺ����ǣ�����Ũ��Ϊ50%��60%��HNO3���ã���Ӧ���ȣ�����Ӧƽ�Ⱥ��ʵ����ȶ�����ȴ��AgNO3���塣��д������ŨHNO3��ϡHNO3��Ӧ�Ļ�ѧ����ʽ��
�ڴӷ�Ӧ����ʽ����ŨHNO3��ϡHNO3�������ã��������������ʸߣ�Ϊʲô��������Ũ��Ϊ50%��60%�����ᣨ��Ũ����
�۹�������ʱʹAg��HNO3���ֹ��������ƣ�����ԭ��
��2����ҵ�ϳ����������ַ����Ʊ��ʣ�
������ʯ����Ҫ�ɷ�Ca3(PO4)2����Ũ����Ϊԭ���Ʊ�������ơ�
�ڹ�����������ں���CaSO4��ʹ�����İٷֺ������͡���������ʯΪ��Ҫԭ���Ʊ�����CaSO4���������ơ�
����ʯ����Ҫ�ɷ�ΪCa5(PO4)3F��������Ũ���������Ʊ�������ơ�
���û�ѧ����ʽ��ʾ�������ַ����Ʊ��ʵĹ��̣�����������ȱ�㡣
��1����Ag+2HNO3(Ũ)====AgNO3+NO2��+H2O
3Ag+4HNO3(ϡ)====3AgNO3+NO��+2H2O
��ϡHNO3�������ʸߣ���̫ϡ��HNO3������Ӧ����̫����
����������Ϊ�����ƣ���ʹHNO3��ַ�Ӧ��������������ͨ�����˻��ա�
��2���仯ѧ����ʽ����Ϊ��
��Ca3(PO4)2+2H2SO4(Ũ)![]() Ca(H2PO4)2+2CaSO4
Ca(H2PO4)2+2CaSO4
���ۣ����������к�CaSO4,ʹ�ĺ������͡�
��Ca3(PO4)2+4H3PO4![]() 3Ca(H2PO4)2
3Ca(H2PO4)2
���ۣ�����������ֻ��Ca(H2PO4)2���ĺ����ϸߡ�
��2Ca5(PO4)3F+7H2SO4(Ũ)![]() 3Ca(H2PO4)2+7CaSO4+2HF��
3Ca(H2PO4)2+7CaSO4+2HF��
���ۣ��˷��Ƶõ������ĺ����Ȣٸ��ͣ����о綾��HF�����������Ⱦ������

 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
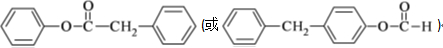
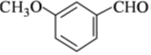
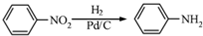 ������
������ �Ǻϳɿ���ҩ���������м��壬��д����
�Ǻϳɿ���ҩ���������м��壬��д����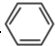 ��
�� Ϊԭ���Ʊ��û�����ĺϳ�·������ͼ
Ϊԭ���Ʊ��û�����ĺϳ�·������ͼ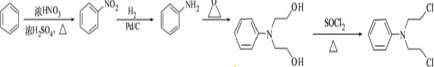

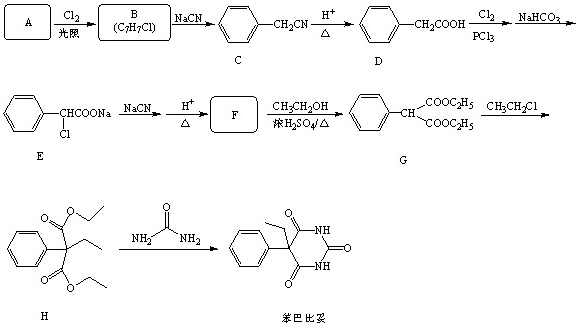


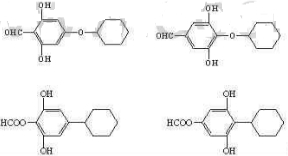

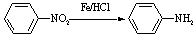 ���������ױ�������
���������ױ������� �ĺϳ�·������ͼ(���Լ�����)���ϳ�·������ͼʾ���磺
��
�ĺϳ�·������ͼ(���Լ�����)���ϳ�·������ͼʾ���磺
��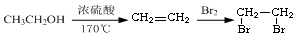

 ������
������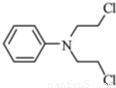 �Ǻϳɿ���ҩ���������м��壬��д����
�Ǻϳɿ���ҩ���������м��壬��д����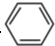 ��
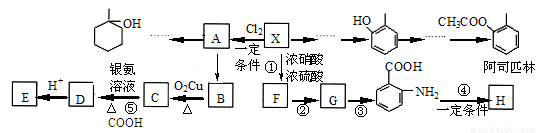
�� Ϊԭ���Ʊ��û�����ĺϳ�·������ͼ �����Լ����ã���
Ϊԭ���Ʊ��û�����ĺϳ�·������ͼ �����Լ����ã���