��Ŀ����
��2013?����һģ�����ͱ�����һ�ֳ������ʹ���ҩ���ҵ�ϳ�ͨ������;���ϳɣ�
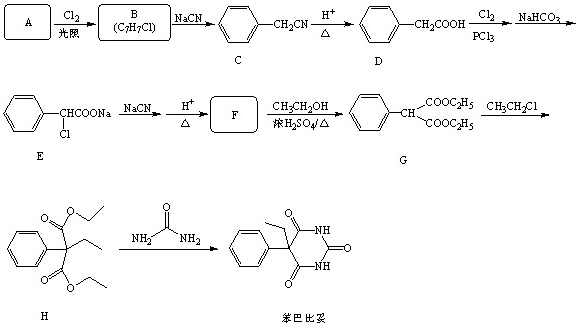
��ش��������⣺
��1��B�Ľṹ��ʽ��
 �����ͱ��ķ���ʽ��
�����ͱ��ķ���ʽ��
��2��G��H�ķ�Ӧ������
��3��������H�ĺ˴Ź���������
��4��д��F��G�����Ļ�ѧ��Ӧ����ʽ
 ��
��
��5��������G�ж���ͬ���칹�壬д��4����������������ͬ���칹��Ľṹ��ʽ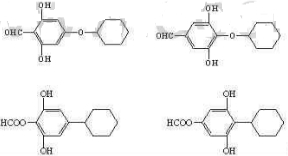
 ��
��
���ܷ���������Ӧ��
��1mol����������������Na��Ӧ�ų�1mol H2��
�����ڷ����廯�����������Ԫ̼���ṹ�����б����ϵ�һ�����ֻ��һ�֣�
��6�����������ϳ�·��������Ҵ�Ϊԭ���Ʊ���HOOCCH2CH2COOH���ĺϳ�·�ߣ����Լ���ѡ���úϳ�·������ͼ��ʾ��
 ��
��
ʾ����CH2=CH2
CH3CH2Br
CH3CH2OH��

��ش��������⣺
��1��B�Ľṹ��ʽ��


C12H12N2O3
C12H12N2O3
����2��G��H�ķ�Ӧ������
ȡ����Ӧ
ȡ����Ӧ
����3��������H�ĺ˴Ź���������
7
7
��壮��4��д��F��G�����Ļ�ѧ��Ӧ����ʽ


��5��������G�ж���ͬ���칹�壬д��4����������������ͬ���칹��Ľṹ��ʽ


���ܷ���������Ӧ��
��1mol����������������Na��Ӧ�ų�1mol H2��
�����ڷ����廯�����������Ԫ̼���ṹ�����б����ϵ�һ�����ֻ��һ�֣�
��6�����������ϳ�·��������Ҵ�Ϊԭ���Ʊ���HOOCCH2CH2COOH���ĺϳ�·�ߣ����Լ���ѡ���úϳ�·������ͼ��ʾ��


ʾ����CH2=CH2
| HBr |
| NaOH |
| ˮ/�� |
��������C��̼���ṹ���ϳ����̿�֪��AΪ�ױ���BΪ ��E����ȡ�����ữ������F��FΪ
��E����ȡ�����ữ������F��FΪ ��Ȼ�����л���Ľṹ�����������
��Ȼ�����л���Ľṹ�����������
 ��E����ȡ�����ữ������F��FΪ
��E����ȡ�����ữ������F��FΪ ��Ȼ�����л���Ľṹ�����������
��Ȼ�����л���Ľṹ���������������⣺��C��̼���ṹ���ϳ����̿�֪��AΪ�ױ���BΪ ��E����ȡ�����ữ������F��FΪ
��E����ȡ�����ữ������F��FΪ ��
��
��1��BΪ ����ͼ�еĽṹ��ʽ��֪���ͱ��ķ���ʽ��C12H12N2O3��
����ͼ�еĽṹ��ʽ��֪���ͱ��ķ���ʽ��C12H12N2O3��
�ʴ�Ϊ�� ��C12H12N2O3��
��C12H12N2O3��
��2��G��H�ķ�Ӧ�У�-CH�ϵ�H���һ�ȡ������÷�ӦΪȡ����Ӧ���ʴ�Ϊ��ȡ����Ӧ��
��3���ɽṹ�ĶԳ��Կ�֪��H�к�7��λ�õ�Hԭ�ӣ���H�ĺ˴Ź���������7��壬�ʴ�Ϊ��7��
��4��F��G�����Ļ�ѧ��Ӧ����ʽΪ ��
��
�ʴ�Ϊ�� ��
��
��5��������G��ͬ���칹�壬���ϣ�
���ܷ���������Ӧ����-CHO��
��1mol����������������Na��Ӧ�ų�1molH2����2��-OH��
�����ڷ����廯�����������Ԫ̼���ṹ�����б����ϵ�һ�����ֻ��һ�֣�����ֻ��һ��H��
����ͬ���칹��Ľṹ��ʽΪ ��
��
�ʴ�Ϊ�� ��
��
��6�����Ҵ�Ϊԭ���Ʊ���HOOCCH2CH2COOH���ĺϳ�·��Ϊ�Ҵ���ȥ���ӳɡ�ˮ�⡢�������ɣ�����ͼΪ ��
��
�ʴ�Ϊ�� ��
��
 ��E����ȡ�����ữ������F��FΪ
��E����ȡ�����ữ������F��FΪ ��
����1��BΪ
 ����ͼ�еĽṹ��ʽ��֪���ͱ��ķ���ʽ��C12H12N2O3��
����ͼ�еĽṹ��ʽ��֪���ͱ��ķ���ʽ��C12H12N2O3���ʴ�Ϊ��
 ��C12H12N2O3��
��C12H12N2O3����2��G��H�ķ�Ӧ�У�-CH�ϵ�H���һ�ȡ������÷�ӦΪȡ����Ӧ���ʴ�Ϊ��ȡ����Ӧ��
��3���ɽṹ�ĶԳ��Կ�֪��H�к�7��λ�õ�Hԭ�ӣ���H�ĺ˴Ź���������7��壬�ʴ�Ϊ��7��
��4��F��G�����Ļ�ѧ��Ӧ����ʽΪ
 ��
���ʴ�Ϊ��
 ��
����5��������G��ͬ���칹�壬���ϣ�
���ܷ���������Ӧ����-CHO��
��1mol����������������Na��Ӧ�ų�1molH2����2��-OH��
�����ڷ����廯�����������Ԫ̼���ṹ�����б����ϵ�һ�����ֻ��һ�֣�����ֻ��һ��H��
����ͬ���칹��Ľṹ��ʽΪ
 ��
���ʴ�Ϊ��
 ��
����6�����Ҵ�Ϊԭ���Ʊ���HOOCCH2CH2COOH���ĺϳ�·��Ϊ�Ҵ���ȥ���ӳɡ�ˮ�⡢�������ɣ�����ͼΪ
 ��
���ʴ�Ϊ��
 ��
�����������⿼���л���ĺϳɣ�Ϊ�߿��������ͣ���ȷϰ���е���ϢΪ�����Ĺؼ��������л���ṹ�����ʵĿ��飬ע��ϳ������й����ŵı仯����Ŀ�Ѷ��еȣ�

��ϰ��ϵ�д�
 ��У��ʦ������ҵ���Ӻ����Ծ�ϵ�д�
��У��ʦ������ҵ���Ӻ����Ծ�ϵ�д� ȫ�̽��ϵ�д�
ȫ�̽��ϵ�д�
�����Ŀ
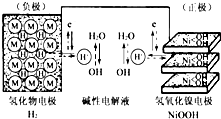 ��2013?����һģ���������г�ʹ�ÿɳ���أ�����ʾ��ͼ��ͼ���⻯��缫Ϊ����������ɿ���H2ֱ�Ӳμӷ�Ӧ��������̫���ܷ��巢�磬��һ���ֵ����������������ҹ�������ع��磮����˵����ȷ���ǣ�������
��2013?����һģ���������г�ʹ�ÿɳ���أ�����ʾ��ͼ��ͼ���⻯��缫Ϊ����������ɿ���H2ֱ�Ӳμӷ�Ӧ��������̫���ܷ��巢�磬��һ���ֵ����������������ҹ�������ع��磮����˵����ȷ���ǣ�������