��Ŀ����
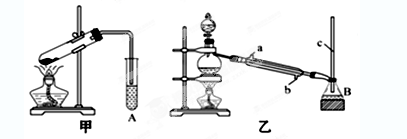
ij��ѧС���������������������װ�ã�����ͼ�����Ի������Ʊ�����ϩ
| | �ܶȣ�g/cm3�� | �۵㣨�棩 | �е㣨�棩 | �ܽ��� |
| ������ | 0.96 | 25 | 161 | ������ˮ |
| ����ϩ | 0.81 | -103 | 83 | ������ˮ |
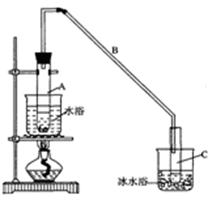
��A�����Ƭ�������� �� ������B���˵�������е������� �� ��
���Թ�C���ڱ�ˮԡ�е�Ŀ���� �� ��
��2���Ʊ���Ʒ��
�ٻ���ϩ��Ʒ�к��л������������������ʵȡ����뱥��ʳ��ˮ�������á��ֲ㣬����ϩ�� �� �㣨���ϻ��£�����Һ���� �� �����ţ�ϴ�ӻ���ϩ��
A.KMnO4��Һ B.ϡH2SO4 C.Na2CO3��Һ D.NaOH��Һ
���ٽ�����ϩ����ͼװ��������ȴˮ�� �� ����f��g���ڽ��룬����ʱҪ������ʯ�ң�Ŀ���� �� ��
���ռ���Ʒʱ�����Ƶ��¶�Ӧ�� �� ���ң�ʵ���ƵõĻ���ϩ��Ʒ�����������۲��������ܵ�ԭ���� �� ��
A.����ʱ��70�濪ʼ�ռ���Ʒ B.������ʵ����������
C.�Ʊ���Ʒʱ���������Ʒһ������
��3���������ֻ���ϩ��Ʒ�ʹ�Ʒ�ķ�������ķ����� �� ��
A.����KMnO4��Һ B.�ý����� C.�ⶨ�е� D.������Ȼ�̼��Һ
��1���� �����У����� �ڷ�ֹ����ϩ�ӷ�����������ϩ
��2���� �ϣ� CD �� g����ˮ �� 830C��C��3��B
���������������1���ٸ�������ϩʵ���֪ʶ������װ��A�����Ƭ�������Ƿ�ֹ���У��������ɵĻ���ϩ�ķе�Ϊ83�棬Ҫ�õ�Һ̬����ϩ������B���˵���������������ã����ڻ���ϩ������
�ڱ�ˮԡ��Ŀ���ǽ��ͻ���ϩ�������¶ȣ�ʹ��Һ����
��2���ٻ���ϩ�������Ȼ�����Һ�����ܶȱ�ˮС���ֲ��ϩ���ϲ㣬���ڷ�Һ��ϩ��Ʒ�л�������������ͻ��������ᴿ����ʱ��c��Na2CO3��Һ��ϴ�ӿɳ�ȥ�
��Ϊ����������Ч������ȴˮ���¿ڣ�g�����룬��ȴˮ�������γ�����������Ч�����ã������������ܳ���ˮ���Է�������ڴ���������ʹ���������ѣ�
�۸��ݱ������ݿ�֪����ֻ���ϩ�ķе�Ϊ83�棻
a������ǰ�ռ�����Ʒ�л������ʣ�ʵ�ʲ����������۲�����
b����ȡ�Ļ���ϩ���ʵ�������ʵ���ƵõĻ���ϩ��Ʒ�����������۲�����
c���ֲ�Ʒ�л��л����������²ⶨ���ĵĻ������������ƵõĻ���ϩ��Ʒ�����������۲�����
��3�����ݻ����û�й̶��ķе㣬���������й̶��ķе㣬�ݴ˿��жϲ�Ʒ�Ĵ��ȡ�
���㣺�����������������Ʊ�ʵ�顢Һ�������ʵ�������

 ��ѧȫ��������ѵ��ϵ�д�
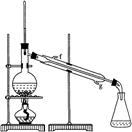
��ѧȫ��������ѵ��ϵ�д�������������Ҫ���л��ϳ��м��壬�㷺Ӧ���ڻ�ѧ��ҵ��ʵ�����Ʊ����������Ļ�ѧ����ʽ��CH3COOH��C2H5OH CH3COOC2H5��H2O��Ϊ֤��Ũ�����ڸ÷�Ӧ�����˴��������ռ������ã�ijͬѧ������ͼ��ʾװ�ý����������ĸ�ʵ�飬ʵ�鿪ʼ���þƾ�����3 min���ټ���ʹ֮����3 min��ʵ������������Թܢ��ٲ��л���ĺ�ȣ�ʵ���¼���£�
CH3COOC2H5��H2O��Ϊ֤��Ũ�����ڸ÷�Ӧ�����˴��������ռ������ã�ijͬѧ������ͼ��ʾװ�ý����������ĸ�ʵ�飬ʵ�鿪ʼ���þƾ�����3 min���ټ���ʹ֮����3 min��ʵ������������Թܢ��ٲ��л���ĺ�ȣ�ʵ���¼���£�
| ʵ���� | �Թܢ��е��Լ� | �Թܢ����Լ� | ����л���ĺ��/cm |
| A | 2 mL�Ҵ���2 mL���ᡢ1 mL 18 mol��L��1Ũ���� | ����̼������Һ | 5.0 |
| B | 3 mL�Ҵ���2 mL���� | 0.1 | |
| C | 3 mL�Ҵ���2 mL���ᡢ6mL 3 mol��L��1���� | 1.2 | |
| D | 3 mL�Ҵ���2 mL���ᡢ���� | 1.2 |
��1��ʵ��D��Ŀ������ʵ��C����գ�֤��H����������Ӧ���д����á�ʵ��D��Ӧ��������������Ũ�ȷֱ���________mL��________mol/L��
��2������ʵ��________����ʵ���ţ������ݣ������Ʋ��Ũ�������ˮ����������������IJ��ʡ�Ũ�������ˮ���ܹ���������������ʵ�ԭ����_______________________________________________________________��
��3������������������������IJ��ʣ���ʵ�鷢���¶ȹ������������IJ��ʷ������ͣ����ܵ�ԭ����____________________________________________������������ɣ���
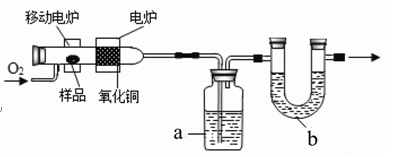
����ˮ�����׳ư�˾ƥ�֣���һ����ʷ�ƾõĽ�����ʹҩ���ϳ�ԭ���ǣ�
�����Ϊ�ᴿԭ������Ϊ�˳�ȥ�÷�Ӧ�ĸ����ˮ������������ˮ����ˮ����������ˮ�������;ۺ���ȡ�ˮ���ᣨ�۵�158�棩������ˮ���ᣨ�۵�135�棩������ˮ����������ˮ���������������������ǣ�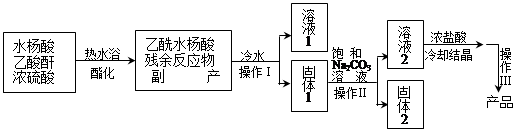
��1������I��III�������� ��ʹ����ˮԡ���ȵ�ԭ���� ��
��2������ѭ�����õ��� ������2�ijɷ��� ��
��3��Ϊ�˼����Ʒ���Ƿ���ˮ���ᣬ�����о��ƣ���ȥ��Ʒ�е�������Ӧ���������������²�����������±���
| ��� | ���� | ���� | ���� |
| �� �� | ȡ������Ʒ����Ͷ��װ������ˮ���Թ��У��� | ����ɫ��Һ | |
| ���Թ��е��� ��Һ | . | ��Ʒ����ˮ���� | |
| �� �ᾧ | ���ֲ�Ʒ����������ˮ�У�ˮԡ���ȣ����ȹ��ˣ�����Һ ������ | �о������� | ����Ʒ |
��4�����кͷ��ⶨ��Ʒ���ȣ�
ȡa g��Ʒ�ܽ���V1 mL1mol/L��NaOH��Һ�У�����ʹ����ˮ����ˮ�⣬����1 mol/L������ζ�������NaOH�����ζ��յ�ʱ��������V2 mL���������Ʒ����Ϊ ��ֻ���г��������ʽ�����ػ�������ˮ���������Ϊ180����
ͨʽΪCnH2n-2��һ����̬����ȫȼ�պ�����CO2��H2O�����ʵ���֮��Ϊ4��3������������״ͬ���칹���У� ��
| A��2�� | B��3�� | C��4�� | D��5�� |
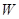 ��һ���л��ϳ��м��壬�ṹ��ʽΪ��HOOC��CH=CH��CH=CH��COOH��
��һ���л��ϳ��м��壬�ṹ��ʽΪ��HOOC��CH=CH��CH=CH��COOH��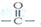 Ϊƽ����ƽ��ṹ����W����������� ��ԭ����ͬһƽ���ڡ�
Ϊƽ����ƽ��ṹ����W����������� ��ԭ����ͬһƽ���ڡ�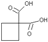 ����һ��ҩ���м��壬���ںϳɿ���ҩ������˳����Q��W��ϵ����������ȷ����
����һ��ҩ���м��壬���ںϳɿ���ҩ������˳����Q��W��ϵ����������ȷ���� 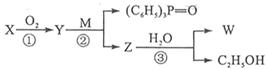
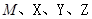 �ֱ����һ���л���ϳɹ�������������ͷ�Ӧ��������ȥ��
�ֱ����һ���л���ϳɹ�������������ͷ�Ӧ��������ȥ��